Microb Newbie Training Materials - Total Number of Colonies
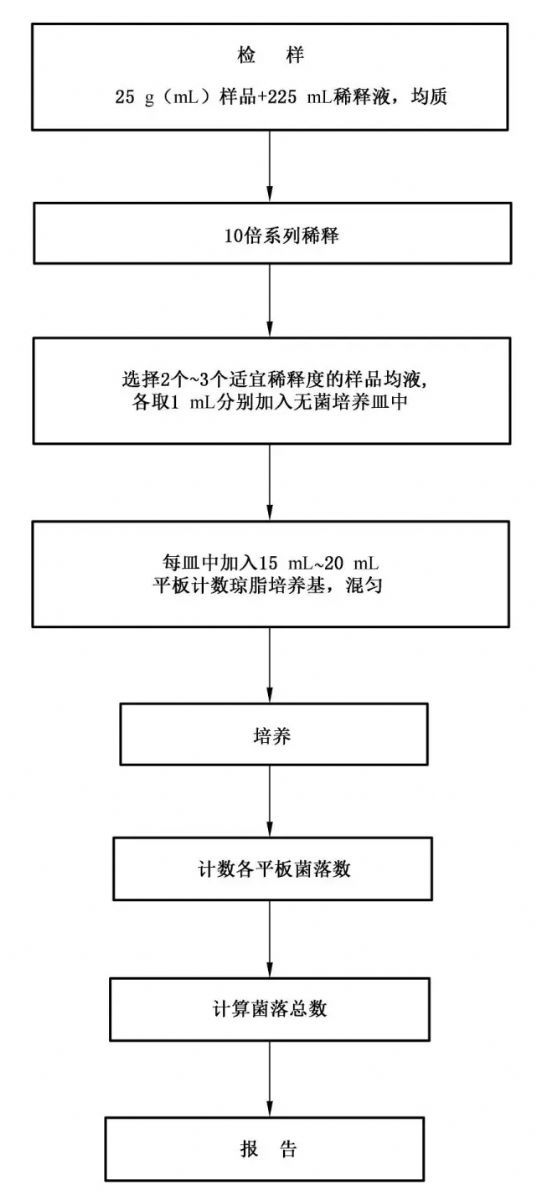
1. Standards used for the total number of colonies Two standards for the total number of colonies used by food companies. (Of course there are other standards) National food standards: GB 4789.2-2016 "Food safety national standard food microbiology test colony total determination" Production water: GB-T5750.12-2006 "Microbiological indicators for standard test methods for drinking water" According to these two standards, their detection methods are similar. The main difference is that the medium is different and the state of the sample is different (solid and liquid in food, liquid only in drinking water). The medium for food is PCA, and the use of production water is NA. 2. Medium composition PCA (plate count agar medium) Tryptone 5.0g Yeast Extract 2.5g Glucose 1.0g Agar 15.0g Distilled water 1000mL Note: Tryptone provides a source of carbon and nitrogen; yeast extract provides a B vitamin; glucose provides energy; agar is a coagulant for the medium. NA (nutrition agar) Peptone 10g Beef extract 3g Sodium chloride 5g Agar 14g (standard 10g-20g) Distilled water 1000mL Note: Peptone and beef extract provide nitrogen, vitamins, amino acids and carbon sources; sodium chloride maintains a balanced osmotic pressure; agar is a coagulant for the medium. 3. Detection method 4, operation attention to details 1 The total number of colonies is a relatively common microbiological test item, and it is also a relatively easy to do microbiological test method, mainly paying attention to aseptic operation and uniformity of diluent. 2 When there is a colony in the blank, you need to analyze which step has a problem, and the man-made method loop . Of course, when the colony appears in the blank, the total number of colonies of the batch is discarded. In the preparation and sample preparation, it is necessary to carry out under the 100-level environment (100-level clean workbench). 3 The uniformity of the dilution, this will affect the results. At the beginning, the two results of the same gradient may be a little different. This is because the diluent is not evenly distributed. If the two gradients have no gradient, then it is not good to do the dilution. This is all done by more, and the technology will improve. (Of course, there is a case where the sample is added with bacteriostatic substances, so there is no gradient.) 5, counting 1 Visually observe, if necessary, with a magnifying glass or colony counter Galaxy 330, record the dilution factor and the corresponding number of colonies. Colony counts are expressed in colony-forming units (CFU). Colony counter Galaxy330/Galaxy331, click here: http://?sid=196 2 Select the number of colonies between 30CFU ~ 300CFU, no colony growth spread plate count of the total number of colonies. Plates below 30 CFU record the number of specific colonies, and more than 300 CFU can be recorded as uncountable. The number of colonies per dilution should be the average of two plates. 3 When one of the plates has larger flaky colonies, it should not be used, but the plate with no flaky colonies should be used as the number of colonies of the dilution; if the flaky colonies are less than half of the plate, the other half of the colonies The distribution is very uniform, you can calculate half a plate and multiply by 2, representing the number of plate colonies. (This is a question that many novices will ask) 4 When there is a chain-like growth between colonies without obvious boundaries, each single strand is counted as one colony. Counting (introduced in the standard) 1 If the number of colonies on only one dilution plate is within the appropriate counting range, calculate the average of the number of colonies on both plates, and multiply the average by the corresponding dilution factor as the total number of colonies per g (mL) sample. 2 If there are two consecutive dilutions of plate colonies within the appropriate counting range, calculate according to formula (1): 3 If the number of colonies on all dilution plates is greater than 300 CFU, the plate with the highest dilution is counted, and the other plates can be recorded as more than uncountable. The results are calculated by multiplying the average number of colonies by the highest dilution factor. 4 If the number of all the dilution plate culture less than 30CFU, the average number of colonies should be the lowest dilution multiplying the dilution factor calculated. If all 5 dilutions (including liquid sample stock solution) had no colony growth plates, places a minimum of less than 1 is multiplied by the dilution factor calculated. 6 If the number of plate colonies of all dilutions is not between 30 CFU and 300 CFU, and some of them are less than 30 CFU or more than 300 CFU, the average number of colonies closest to 30 CFU or 300 CFU is multiplied by the dilution factor. 6, the problem of the total number of colonies (some questions asked by novices) 1. Some new people may take the plate with the total number of colonies to ask others what is this? A: This is not visible. The total number of colonies can only detect the hygienic condition of the sample. The number of bacteria in one sample cannot be verified. This is a misunderstanding that should not be committed. 2. If the number of plate colonies in all dilutions is not between 30 CFU and 300 CFU, some of which are less than 30 CFU or greater than 300 CFU. Someone will not know how to judge that the gradient is closer to 30-300. Example: a gradient: 28/28; another gradient: 305/305, then what is the data closer? Two methods (provided by netizens): a, according to the standard literal meaning directly after the difference comparison and then multiplied by the dilution factor: 28-30 = -2, they differ by 2; 305-300 = 5, they differ by 5. 2 is smaller than 5, then take 28 corresponding gradient Multiply by the dilution factor; b. Restore to the same dilution to make a difference: convert the two gradients into the same gradient, generally convert 28 into 280. 280-300=-20, the difference is 20; 305-300=5, the difference is 5. 20 to 5. To be big, then take the corresponding gradient of 305. (Personal opinion: I am more biased towards the second, because the comparison of the same gradient can reflect the reliability of the data, and I prefer to use the number of the previous gradient, then this value can be reduced by one step Error, the data may be closer to the sample) 3. Sometimes there is a situation where one colony appears on one plate in the lowest dilution gradient and the other grows aseptically. So how is the result? A: (The solid state is an example) It has also been discussed in the past. Some people think that the report is written in 5, and some reports that the report is written as <10 (compared to the time when both plates are aseptically grown <10) are actually applicable. . (I use the first one; personal understanding is not long report <10, so long is also reported <10? Not reasonable) 4. When doing the total number of colonies, sometimes the samples and colonies can not be distinguished (the older generation will not do this), then how to distinguish between the two substances? A: TTC is added to the medium (generally 2% concentration 1mL is added to 400mL of medium, the concentration of TTC is not too high, there will be inhibition). The principle of TTC: TTC and live cell mitochondrial succinate dehydrogenase reaction, the formation of red a month. Then the growing colonies turn red, so you can distinguish between colonies and samples. There is also a method: 4 ° C refrigerated to store a sample and medium mixed with the plate as a control, observe the morphological characteristics of the colonies, the older generations basically rely on this to distinguish. 5. Does the total number of colonies include mold? A: The concept of the total number of colonies is as specified. After the food samples are processed and cultured under certain conditions (such as culture medium, culture temperature, culture time, etc.), the total number of microbial colonies formed per g (mL) sample is obtained. . Therefore, microorganisms such as mold and yeast grown on PCA are also included. 6. Is the medium temperature not well controlled? A: The premise is that the medium is sterilized and placed in a water bath for 46 degrees Celsius. At the time of use, a colleague took the medium from the water bath to the transfer window, sprayed alcohol on the surface of the medium, and then turned on the UV light of the transfer window for 5 minutes (this step is to sterilize the surface of the medium to reduce pollution to the clean room). The colleague inside took it up after 5 minutes and put it on the palm of the hand. The body is not hot, so the temperature is best to pour the tablet. (Note: the speed of the inverted plate should be fast. It is best to take only one bottle at a time to avoid solidification of the medium) 7. Some friends do not understand the meaning of the unit CFU of the total number of colonies. A: CFU is the abbreviation of Colony-Forming Units, which means the number of colonies formed colonies, is not equal to the number of bacteria. (For example, if two identical bacteria are close together or attached together, then the two bacteria will form a colony, which is 2 bacteria, 1 CFU). The total number of colonies is often the plate count method. After the culture, we count the number of colonies growing on the plate to calculate how many colonies can be cultured per ml or per gram of sample to be tested, so CFU/ml Or CFU/g report. 8. The harm of colony exceeding the standard. A: The total number of colonies of food is seriously exceeded, indicating that the hygienic condition of the products does not meet the basic hygiene requirements, which will destroy the nutrient content of the food, accelerate the spoilage of the food, and make the food lose its edible value. Consumers who consume foods with excessive microbial counts are prone to intestinal diseases such as diarrhea, which may cause symptoms such as vomiting and diarrhea, and endanger human health and safety. However, it should be emphasized that the total number of colonies and pathogenic bacteria are essentially different. The total number of colonies includes pathogenic bacteria and beneficial bacteria. The main damage to human body is the pathogenic bacteria, which will destroy the normal colony environment in the intestine. Some may be killed in the intestines, some will remain in the body causing diarrhea, damage to the liver and other body organs, and beneficial bacteria include lactic acid bacteria often mentioned in yogurt. However, the excessive number of colonies also means that the chances of pathogens exceeding the standard increase, increasing the risk of harm to human health. 9. The culture medium after the culture appears to be dry. A: The chapped is caused by the lack of water in the medium, so when there is a dry crack, first observe the number and location of the dry plate. See if the temperature inside the incubator is unstable (usually a stack of six plates can be placed, and at the same time, it is necessary to keep a little gap between each stack to allow it to ventilate); after removing the cause of the instrument, it is necessary to see if the medium is too Thin (beginners can practice with a triangular bottle of water); there are other reasons, in fact, in the case of chapped, the result is invalid (unless the chapped condition is not serious). Find out where the problem is, and you can avoid making mistakes next time. 10. Configuration of the medium. A: The packaging of the medium (on the standard) will be written first to dissolve the heat and then dispense. Is it necessary to cook the heat to dissolve it? First of all, the configuration method on the bottle is to directly configure one liter of PCA, where it is necessary to cook heat to dissolve (prevent unevenness, so that some medium does not solidify). In fact, there are two ways to configure the medium: the first one: configure the medium with a large container, then add water to cook the heat to dissolve (pay attention to stir, to prevent charring), after the dissolution is completed, the packaging is carried out (we need to pay attention to safety), and then To sterilize. The second method is to use a 500mL flask (other containers are also available) to configure 300ml of medium, add water to dissolve slightly (this step does not require heating), and then remove the sterilization. Steroids Powder,Raw Steroid Powder,Steroid Raw Powder,Steroid Protein Powder Shaanxi Hongbaiyi Biotech Co., Ltd. , https://www.sxhongbaiyi.com