ChemBioChem: "Mature eggs are born?!" - Let's take a look at science
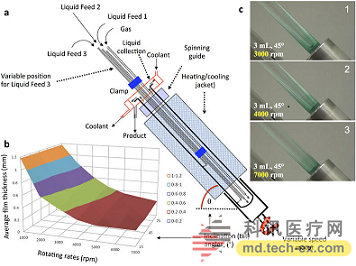
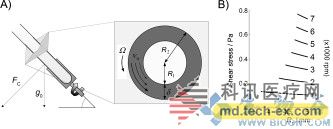
Release date: 2015-02-03 A recent study published in ChemBioChem on the reduction of cooked denatured proteins has caused heated discussions. Real research is a breakthrough in protein folding reduction technology, and the results are significant, but not so exaggerated. The University of California, Irvine, and the Australian Flinders chemists have jointly developed a Vortex Fluid Device (VFD) that restores the activity of the cooked protein's lysozyme to 80% of the uncooked protein. (The lysozyme protein denaturation after cooking: the spatial conformation is altered, its physical and chemical properties are altered and the biological activity is lost). This technology seems to have no special significance. In fact, this will save more than tens of billions of dollars in costs for industries, pharmaceuticals, agriculture, and environmental companies that focus on protein production. Experimental device (picture from the network) VFD internal map (picture from the network) VFD seems inconspicuous, but everything is carefully designed by researchers. The glass tube of 10 mm wide and 16 cm long is inclined at an angle of 45 degrees, and the rotational speed reaches 5000 rpm during operation. During high-speed rotation, the liquid sample in the tube will form a fluid film that is only a micron thick, flowing together in the same direction following the glass tube. This meticulous design makes the sample in the tube produce a velocity gradient. Although it is only a micron thick fluid film, the closer to the liquid in the tube wall, the closer the speed is to the rotation speed of the glass tube, and the speed near the tube is slightly weaker. Shear Stress is produced. It is this shearing force that causes the protein to open and refold to be restored. The liquid sample has a shear force of approximately 0.5246-0.5574 Pa in a VFD at 5000 rpm, which is just suitable for protein structure opening. Of course, for different protein structures or sizes, the VFD settings include speed, run time, etc. will change. (Image from research paper) The researchers first experimented with natural lysozyme (HEWL) in chicken protein. After heating the egg whites at 90 degrees for 20 minutes, the researchers dissolved the solid egg whites into the urea and placed them in a VFD rotation. Only after 2.5 minutes, the researchers detected a recovery in the activity of HEWL, indicating a structural refolding reduction of HEWL. The researchers also tested recombinant HEWL, purified recombinant caveolin-1, and protein kinase A. Although these molecules were different in size, the researchers found the optimal VFD speed that would restore the activity of the inactivated protein. Conditions such as time, buffer type and protein concentration. However, the researchers also said that this technique is more suitable for the folded proteins extracted from inclusion bodies, because they found that when using cooked egg white as a sample to restore the HEWL active structure, much of the mechanical energy is distributed to other miscellaneous proteins in the egg white. On, the recovery efficiency is reduced. In this regard, VFD is actually more suitable for specific protein industry or scientific research, such as antibody companies, pharmaceutical companies. Many industries now produce recombinant proteins. For example, the production of antibodies involves the use of engineered bacteria to carry specific recombinant genes, large amounts of replication and expression of specific antibodies, and later collection and purification. However, in this process, because of the lack of a specific factor or environmental discomfort, recombinant proteins often fail to fold correctly and densely accumulate to form inclusion bodies. Restoring these misfolded proteins has been a headache for related industries. Therefore, some special antibodies for the treatment of cancer cells must use very expensive and harsh conditions to avoid misfolding of proteins. The invention of this VFD is definitely the gospel of the protein production industry. It can recover the wrong protein folding in a few minutes, greatly reducing the time and cost of protein production, and making some critical antibodies can be produced under ordinary conditions. Not only will people who produce recombinant proteins gain higher profits, but some related cancer drug treatments may therefore be more affordable. Currently, the University of California, Irvine has applied for a patent for the technology and will soon be on the market. Source: Bio Valley Collagen is the most abundant protein in mammals, accounting for 25% to 30% of the total protein. It is widely present in all tissues from the body surface of lower vertebrates to mammalian bodies. Collagen monomers are long cylindrical proteins with a length of about 280 nm and a diameter of 1.4–1.5 nm. It consists of 3 polypeptide chains that are supercoiled around each other. Collagen,Collagen Peptides,Collagen costco,Collagen Peptide Products YT(Xi'an) Biochem Co., Ltd. , https://www.ytwholefood.com