The basic principle of glycosylation modification
For the organic food market, we have created a range of unique high-quality starch and sugar. All of Our ingredients are certified to applicable organic standards: National Organic Program (NOP) and European Union (EU), in order to ensure that your product benefits from optimal positioning, whether you operate on a global or local scale, we`ll work to keep you ahead of trends and improve your profitability. Plant Starch,Organic Potatoes,Organic Corn,Organic Maltodextrin Organicway (xi'an) Food Ingredients Inc. , https://www.organicwayince.com
Second, glycosylation modification function Glycosyltransferase and glycosidase play an important role in the process of glycosylation. As a result of glycosylation, different proteins are labeled differently, changing the conformation of the polypeptide and increasing the stability of the protein.
Third, the glycosylation modification process glycosylation modification mainly occurs in the endoplasmic reticulum and Golgi apparatus. The main process is to transfer the sugar group to the protein under the action of glycosyltransferase, and form a glycosidic bond with the amino acid residue on the protein, after a series of transport, sugar chain end shearing, modification and fucosylation or saliva. Acidification and other complete glycosylated protein assembly 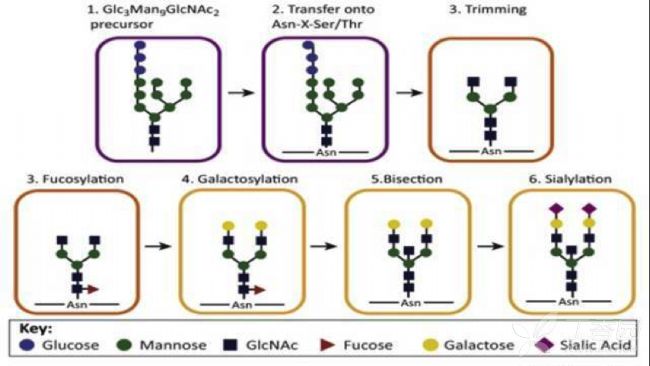
Glycosylation Modifications The types of glycosylation of proteins in mammals can be divided into two main types: N-glycosylation and O-glycosylation. Most glycoproteins contain only one type of glycosylation. However, some protein polypeptides have N-glycan and O-glycan chains at the same time.
N-glycosylation
The N-glycan is referred to as N-glycosylation by covalent attachment to the free NH2 group of aspartic acid of the protein. N-linked sugar chain synthesis begins in the endoplasmic reticulum (ER) and is completed in the Golgi apparatus. 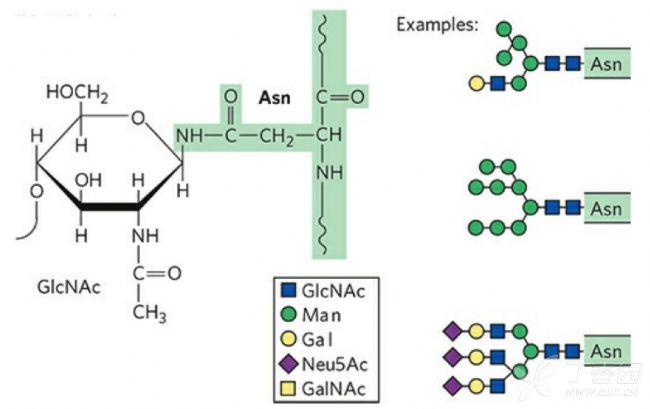
The first step in the synthesis of N-glycans is to add a 14-glycan core oligosaccharide to the asparagine whose characteristic sequence of the newly formed polypeptide chain is Asn-X-Ser/Thr (X stands for any one of the amino acids). Asparagine acts as a sugar chain acceptor. The core oligosaccharide is composed of two molecules of N-acetylglucosamine, nine molecules of mannose and three molecules of glucose. The first N-acetylglucosamine is combined with the phosphate group of the phosphonol phosphate on the ER double lipid membrane. When a new polypeptide is synthesized on the ER membrane, the entire sugar chain is transferred together. After the oligosaccharide is transferred to the nascent peptide, it is further processed in the ER, and three molecules of glucose and one molecule of mannose are sequentially removed. The glycoprotein formed in the ER has a similar sugar chain. After the Cis surface enters the Golgi apparatus, during the transport between the membrane capsules, most of the mannose on the original sugar chain is cleaved, but a variety of glycosyl groups are The transferase sequentially adds different types of sugar molecules to form oligosaccharide chains with different structures. Proteins in body fluids such as plasma often undergo N-glycosylation, so N-glycoproteins are also called plasma glycoproteins.
2. O-glycosylation
The O-glycan is covalently linked to the free OH group of the serine or threonine of the protein. The O-glycosylation site has no conserved sequence, and the sugar chain has no fixed core structure. The composition can be either a monosaccharide or a large sulfonated polysaccharide, so the O-glycosyl group is compared with the N-glycosylation. Analysis will be more complicated. 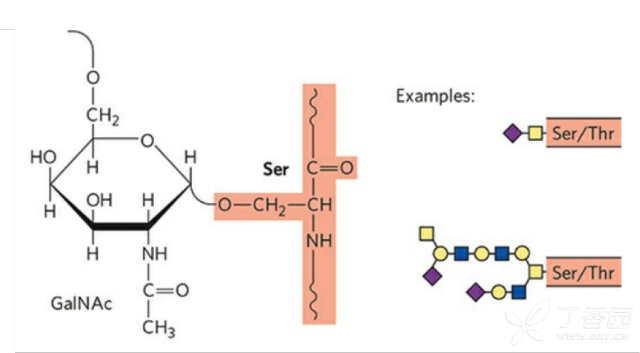
O-glycosylation is carried out in the Golgi apparatus. Usually, the first attached sugar unit is N-acetylgalactose, and the attached site is a hydroxyl group of Ser, Thr or Hyp, and then the sugar residue is successively transferred to form an oligosaccharide. The chain, the sugar donor is also a nucleoside sugar, such as UDP-galactose. O-glycoprotein is mainly found in mucus and immunoglobulin.
