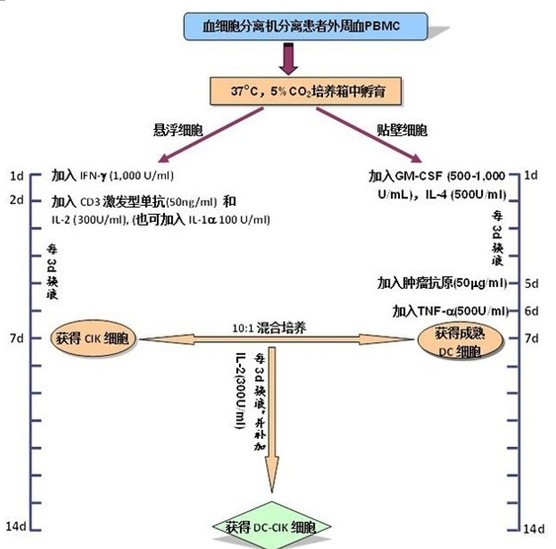
DC-CIK cell preparation method
API Powder is fully defined in ICH Q7A as any substance or mixture of substances intended for use in the manufacture of pharmaceutical products and, when used in pharmaceutical products, as an active ingredient of pharmaceutical products. Such substances have pharmacological activities or other direct effects in the diagnosis, treatment, symptom relief, management or prevention of disease, or can affect the function or structure of the body. The active ingredient of a medicine. Only when an API is processed into a pharmaceutical preparation can it become a medicine for clinical application. alprazolam api powder,pregabalin api powder,buy api powder,api ingredients Shaanxi YXchuang Biotechnology Co., Ltd , https://www.peptide-nootropic.com

CIK is an abbreviation for "Cytokine-Induced Killer Cells", which is referred to as "cytokine-induced killer cells" in Chinese. CIK is a group of heterogeneous cells in which mononuclear cells are cultured under the action of CD3 mAb and various cytokines (including IFN-γ, IL-2, etc.), and CD3+CD56+ cells are the main effector cells. The powerful anti-tumor activity of T lymphocytes has the non-MHC (major histocompatibility antigen) limiting tumor killing ability of NK cells (natural killer cells). CIK cells have high tumoricidal activity, broad spectrum of tumoricidal activity, low toxicity to normal tissues, and high amplification in vitro. They are currently used immunotherapy cells widely used in clinical practice.
DC is the abbreviation of "DendriticCells", which is called "dendritic cells" in Chinese. It is named for its many dendritic or pseudo-protrusion protrusions when it matures. The DC was discovered in 1973 by the 2011 Nobel Prize winner and Canadian scientist RalphM. Steinman. It is the most powerful antigen-presenting cell (APC) currently discovered. It has been confirmed that DC is the only APC that can significantly stimulate the proliferation of naïve T cells, while other APCs (such as monocyte macrophages, B cells, etc.) can only stimulate activated or memory T cells. . DC is the initiator of the body's adaptive T cell immune response and plays an extremely important role in tumor immunity.
DC-CIK, DC and CIK cells, are co-cultured in vitro and then returned to the patient. Strictly speaking, the final effector cells are CIK cells that are activated in vitro by DC. A number of studies have shown that DC and CIK have synergistic effects. After co-incubation, the expression of co-stimulatory molecules on DC surface and antigen-presenting ability are significantly improved, while the proliferative capacity of CIK and cytotoxic activity in vitro and in vivo are also enhanced, so DC- CIK is more effective than CIK alone. Co-culture of tumor antigen-loaded DCs with CIK can stimulate the production of tumor antigen-specific T cells. Such DC-CIK treatment has both specific and non-specific dual tumor killing effects, and DC stimulation without tumor antigen loading. Activated CIK is more active and is often used in clinical and scientific research. 
 GM-CSF (granulocyte macrophage colony-stimulating factor):
GM-CSF (granulocyte macrophage colony-stimulating factor):
GM-CSF is a hematopoietic growth factor that stimulates the formation of colonies of neutrophils and macrophages in vitro and has the function of promoting the proliferation and development of early red megakaryocytes and eosinophils. GM-CSF is one of the first cytokines identified to have an effect on DC.
The function of GM-CSF in DC culture is to promote the differentiation of monocytes into large macrophage-like cells, and the expression of MHC class II molecules on the cell surface is enhanced, thereby enhancing the antigen presentation function of cells. In addition, GM-CSF can also promote the survival of DC.  IL-4 (Interleukin-4)
IL-4 (Interleukin-4)
The role of IL-4 in the induction of DCs by monocytes is to inhibit the overgrowth of macrophages, thereby directing monocytes to differentiate into DCs. If IL-4 is not added to the culture system, monocytes will differentiate into macrophages. At the same time, IL-4 also has the ability to reduce the expression of CD14 molecules on the cell surface. A decrease in the expression level of CD14 is an important marker for the differentiation of monocytes into DCs.
The interaction of GM-CSF and IL-4 can differentiate mononuclear cells into immature DCs. At this time, DCs have strong antigen uptake and processing ability, but antigen presentation ability is weak. MHC class I, class II and B7 family molecules (CD80, CD86, etc.) are moderately expressed on the cell surface, but CD14 is not expressed.  TNF-α (tumor necrosis factor-α)
TNF-α (tumor necrosis factor-α)
TNF-α can down-regulate the macrocytosis of immature DCs and the expression of surface Fc receptors, so that the intracellular MHC class II molecular compartment (class IIcompartment) disappears, but can upregulate the cell surface? MHC class I, class II molecules and B7 The expression of family molecules (CD80, CD86, etc.) differentiates immature DCs into mature DCs (matureDC). At this time, the antigen uptake and processing ability of DCs is significantly weakened, and the antigen presentation ability is significantly enhanced, which can strongly activate T cells. .  CD3-excited monoclonal antibody :)
CD3-excited monoclonal antibody :)
The first signal of T cell activation comes from the receptor on the surface of T cells, that is, the specific binding of T cell antigen receptor (TCR) to the antigen presented by APC, that is, the specificity of T cells to antigen. Identification. TCR is a heterodimer composed of two different peptide chains which, on the surface of T cells, binds to CD3 molecules by non-covalent bonds to form a TCR/CD3 complex. TCR recognizes specific antigens and causes cytoplasmic tails on the surface of CD3 and T cells to co-receptor CD4 or CD8 molecules, thereby activating tyrosine kinases (Lck, Fyn and ZAP-70, etc.) linked to the cytoplasmic tail. To promote tyrosine (Y) phosphorylation in the immunoreceptor tyrosine activation motif (ITAM) of the cytoplasmic region of CD3 molecule. Phosphorylated tyrosine (pY) further phosphorylates downstream tyrosine-containing proteins, causing a cascade of kinase activation (phosphoinositol pathway MAP kinase pathway, etc.), ultimately by activating transcription factors into the nucleus Binding to target genes (such as IL-2 and IFN-γ, etc.) that regulate T cell proliferation and activation, causing gene expression and transcription, and T cells thus transition from a quiescent state to a proliferative and activated state.
It can be seen from the above that CD3 molecules play an extremely critical role in the transduction of T cell activation signals. CD3-excited monoclonal antibody specifically binds to the CD3 molecule on the surface of T cells, which can cause phosphorylation of tyrosine in the ITAM motif of the cytoplasmic region of CD3 molecule, which in turn leads to activation of downstream signals of T cell proliferation and activation, thereby enabling T Cell proliferation and activation. That is to say, CD3-excited monoclonal antibody can mimic the recognition and activation process of antigen and TCR/CD3 complex, which leads to the proliferation and activation of T cells, and is therefore an indispensable stimulating factor in CIK cell culture.
In addition, CD3-excited monoclonal antibodies must pay attention to the clone number when selecting. Studies have shown that only CD3-excited mAbs with the clone number OKT-3 can stimulate the proliferation of T cells in all humans, while CD3-excited monoclonal antibodies of other clones can only stimulate some human T cells. Therefore, in the case of CIK culture, it is best to use OKT-3 clones to ensure that each patient's T cells can be activated.  IL-2 (interleukin-2)
IL-2 (interleukin-2)
IL-2 was originally discovered as T cell growth factor (TCGF) and is the most important cytokine that causes T cell proliferation. IL-2 is both an autocrine cytokine and a paracrine cytokine that promotes T cell activation and enters a cell division state by specific binding to the IL-2 receptor (IL-2R) on the surface of T cells. In addition, IL-2 can also stimulate the growth of NK cells and enhance their killing ability. Therefore, IL-2 must be added to CIK cell culture to promote the proliferation and activation of T cells.  IFN-γ (interferon-γ)
IFN-γ (interferon-γ)
IFN-γ has the effect of up-regulating the expression of IL-2R on the surface of peripheral blood lymphocytes, thus enhancing the sensitivity and intensity of T cells to IL-2 proliferative response. The addition of IFN-γ during the induction of CIK cell formation reduces the amount of IL-2. The study found that the order of IFN-γ addition is closely related to the cytotoxic activity of CIK. The cytotoxic activity of CIK was significantly increased by adding IFN-γ first, and then adding IL-2 after 24 hours of culture.  IL-1a (interleukin-1a)
IL-1a (interleukin-1a)
IL-1a can also mediate up-regulation of IL-2R on the surface of peripheral blood lymphocytes. When IL-1a is combined with IFN-γ and the elicited CD3 monoclonal antibody, the cytotoxic effect of CIK can be significantly enhanced. 
1.2 Lymphocyte separation solution further purification of mononuclear cells (PBMC) by density gradient centrifugation;
1.3 Serum-free medium was washed twice to obtain PBMC with a purity of more than 90%, and the number of cells should be 1-3 x 108.
2. (Optional step) Preparation of tumor antigens]
The tumor antigen for loading DC may be a Tumor-Specific Antigens (TSA) or a Tumor-Associated Antigens (TAA), or may be a tumor whole cell antigen.
DCs loaded with TSA or TAA have a good ability to overcome these drawbacks, since it is not necessary to know that those antigens are TSAs or TAAs of tumor cells, and that multiple different tumor antigens in whole antigens can induce DCs to target different antigenic determinants. The cytotoxic T lymphocytes (CTL) are cloned to achieve effective killing of tumor cells.
There are many methods for tumor cell whole antigen-loaded DC, including loading DC with tumor cell lysate, DC with apoptotic tumor cells, DC with necrotic or dead tumor cells, DC with tumor living cells, and DC with tumor cells. Fusion and so on. At present, it is commonly used in clinical practice to load DC with tumor cell lysate, because the method is simple, rapid and effective.
Repeated freezing and thawing is a common method to obtain tumor cell lysate. The specific steps are as follows:
2.1 Surgical removal of tumor specimens, under sterile conditions, remove necrotic tissue and non-tumor tissue adjacent to the tumor;
2.2 Wash 3 times with sterile saline;
2.3 Cut the tumor tissue with a sterile tissue scissors, add RPMI 1640 medium, and grind thoroughly;
2.4 200 mesh sterile mesh filtration after collection of single cell suspension;
2.5 Resuspend the cells to 1-2 x 107/ml in RPMI 1640 medium and place in a 5 ml sterile cryotube;
2.6 The frozen tube was immersed in liquid nitrogen for quick freezing, taken out after 10 min, and then quickly thawed in a 37 ° C water bath for 10 min. Repeat 3-5 times;
Note: It can also be freeze-thawed 3-5 times at -80 °C / 37 °C.
2.7 Add the tumor lysate to the centrifuge tube, centrifuge at 3000 rpm for 10 min;
2.8 Collect the supernatant, filter and sterilize by 0.22μm filter, and check the protein content and bacteria, fungi and mycoplasma;
Store at 2.9 -80 °C for backup.
3.0 CIK cell culture and identification 3.1 PBMC obtained in step 1 The cell concentration was adjusted to 2 x 106/ml with serum-free medium and placed in a culture flask;
3.2 Incubate for 2 h at 37 ° C in a 5% CO 2 incubator to allow monocytes to adhere;
3.3 collecting suspended cells, adjusting the cell concentration to 1-2 x 106/ml with serum-free medium;
3.4 adding 1,000 U/ml recombinant human IFN-γ culture;
3.5 After 24 h, 50 ng/ml CD3 monoclonal antibody and 300 U/ml recombinant human IL-2 were added to stimulate the growth and proliferation of CIK cells;
Note: At this time, 100 U/ml recombinant human IL-1a can also be added at the same time.
3.6 Change the liquid or expand the bottle once every 3 days and add the recombinant human IL-2300 U/ml;
3.7 On the 7th day of culture, harvest CIK cells, the number should reach 1x109 or more;
3.8 CIK Cell Quality Control:
3.8.1 trypan blue staining to detect cell viability: live cells should be above 80%;
3.8 .2 The expression of CD3, CD8 and CD56 on the cell surface was detected by flow cytometry, and the proportion of CD3+CD56+ cells was significantly increased.
4. Culture and identification of DC cells
4.2 Change the liquid once every 3d and supplement the cytokines;
4.3 (optional step)? On the 5th day of the culture, add the tumor antigen 50 mg/ml obtained in step 2, and carry on the antigen load on the DC;
Note: If the antigen load is not applied to DC, this step is omitted.
4.4 On the 6th day of culture, recombinant human TNF-α (500 U/ml) was added to induce DC cell maturation;
4.5 On the 7th or 8th day of culture, DC cells should be harvested in an amount of 1×106 or more;
4.6 DC quality inspection:
4.6.1 Trypan blue staining to detect cell viability: live cells should be above 80%;
4.6.2 Flow cytometry detects the expression of molecules such as HLA-DR, CD83 and CD86 on the surface of DC cells to determine whether DCs are mature.
5.0 Preparation and quality inspection of DC-CIK cells 5.1 Collect DC cells and CIK cells obtained in steps 4 and 3, co-culture in a ratio of 1:10 (number ratio), and add recombinant human IL- in serum-free medium. 2 (300 U/ml);
5.2 Change the amount of liquid once every 3 days and add recombinant human IL-2 (300 U/ml).
5.3 Collect cells on the 7th day, the number of cells should reach 1 × 1010 or more;
5.4 Quality inspection of DC-CIK cells:
5.4.1 trypan blue staining test: live cells should be above 80%;
5.4.2 Flow cytometry to detect the expression of CD3, CD8, CD56 and other molecules on the cell surface: the proportion of CD3+CD56+ cells should be above 20%.
5.4.3 cell killing experiment: DC-CIK cells are used as effector cells, tumor cells (which may be primary tumor cells or tumor cell lines) as target cells, and effector cells and target cells are 10:1 (number ratio) The ratio was added to a 96-well U-shaped plate, each well containing 1 x 104 target cells, a final volume of 200 ml, and three replicate wells. After incubation for 4 h, the culture supernatant was taken, and the killing rate of the effector cells against the target cells was detected by a lactate dehydrogenase (LDH) kit.
5.4 .4 Before harvesting cells, take a small amount of culture for bacterial and fungal culture, and test for mycoplasma, chlamydia, and endotoxin (standard: negative for pathogen detection, endotoxin <5 Eu). 

[2] Zhang Zhiwei, Song Xin. Standardized study of clinical preparation of DC-CIK cells. Chinese Journal of Oncology, 2011; 20(2): 85-88.
[3] Li R, Wang C, et al. Autologous cytokine-induced killercell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012; 61:2125–2133. 
According to its source, apis are divided into chemical synthetic drugs and natural chemical drugs.
Chemical synthetic drugs can be divided into inorganic synthetic drugs and organic synthetic drugs. Inorganic synthetic drugs are inorganic compounds (very few are elements), such as aluminum hydroxide and magnesium trisilicate for the treatment of gastric and duodenal ulcer, etc. Organic synthetic drugs are mainly made of basic organic chemical raw materials, through a series of organic chemical reactions and drugs (such as aspirin, chloramphenicol, caffeine, etc.).
Natural chemical drugs can also be divided into biochemical drugs and phytochemical drugs according to their sources. Antibiotics are generally produced by microbial fermentation and belong to the category of biochemistry. In recent years, a variety of semi-synthetic antibiotics are the combination of biosynthesis and chemical synthesis products. Among apis, organic synthetic drugs account for the largest proportion of variety, yield and output value, which is the main pillar of chemical pharmaceutical industry. The quality of the API determines the quality of the preparation, so its quality standards are very strict. All countries in the world have formulated strict national pharmacopoeia standards and quality control methods for the widely used APIS.
Specific API after processing -- pharmaceutical preparations
The term API is used mainly in relation to the finished product. Mainly raw materials obtained by chemical processing means, supply raw materials for the production of finished drugs. For example, cefpirome sulfate for injection is a drug, then cefpirome sulfate is an API
