Surfactant micelle characterization using dynamic light scattering
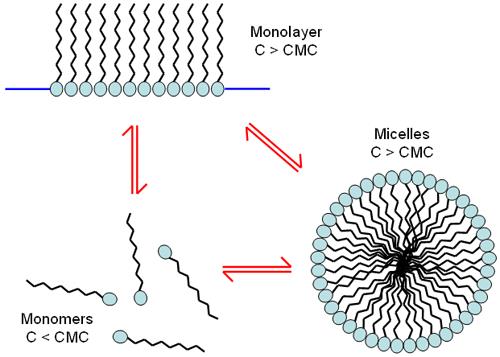
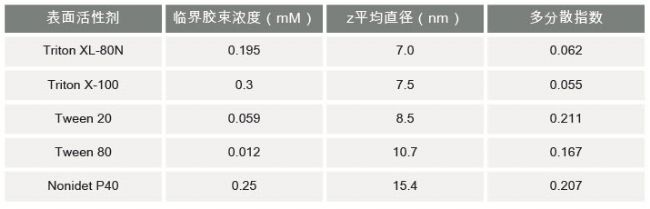
Introduction Surfactants are generally classified according to the type of end groups [2]: The ionic surfactant is absorbed to the surface to generate an electrical charge. The cationic surfactant will cause the surface to be positively charged and the anionic surfactant will cause the surface to be negatively charged. Nonionic surfactant molecules are uncharged in aqueous media, but generally contain highly polar components such as polyoxyethylene groups. The amphoteric surfactant may have a negative or positive charge depending on the pH of the solution. At low concentrations, the surfactant molecules are non-associated monomers. As the concentration of the surfactant increases, the attractive force and repulsive force between the molecules can form a self-assembly phenomenon, resulting in a monolayer film structure or micelle formation (Fig. 1). The concentration at which micelles are formed is referred to as "critical micelle concentration" (CMC). The properties of the micelles can be controlled by subtle changes in the chemical structure of the surfactant molecules or by changing the conditions of the dispersed phase. It is well known that changes in pH, ionic strength and temperature can affect the particle size and shape of the surfactant micelles. In some cases, the particle size of the micelles may be affected by the concentration of the surfactant. Figure 1: Surfactants can exist in different phases depending on the concentration of the sample Researchers have used a variety of techniques to study surfactant micelles [3-5]. This application note explores the application of light scattering techniques in various aspects of surfactant characterization. Dynamic Light Scattering (DLS) is a technique used for particle size detection of samples, especially submicron samples. This technique detects fluctuations in the intensity of scattered light emitted by particles undergoing random Brownian motion over time. Analysis of these intensity fluctuations yields a diffusion coefficient such that the particle size can be obtained by the Strokes-Einstein equation. The traditional DLS instrument uses a 90o detection angle. The sensitivity of this optical configuration may not be sufficient to ensure successful measurement of surfactant micelles. The Zetasizer Nano instrument family uses non-invasive backside scattering (NIBS) optics [6-8] to detect scattered light at 173°. This novel optical design maximizes scattered light detection while maintaining signal quality. This provides the high sensitivity required to measure nanoparticle size (eg, surfactant micelles) at low concentrations. Experiment All measurements reported in this application were performed using a Zetasizer Nano S at 25oC. The Nano S features a 4mW He-Ne laser operating at 633nm and an avalanche photodiode (APD) detector. result Particle size characterization of surfactant micelles Particle size measurements of various surfactant micelles were performed on a Zetasizer Nano S. Table 1 summarizes the Z average diameter (unit: nanometer) and polydispersity index values ​​for each sample. The micelle concentration prepared was twice the critical micelle concentration of the surfactant. The Z average diameter refers to the average hydrodynamic diameter, and the polydispersity index refers to an estimate of the distribution width. These parameters are calculated according to the international standard ISO 13321 using dynamic light scattering technology [9]. Table 1: Z average diameter (unit: nanometer) and polydispersity index values ​​for various surfactant micelles measured using a Zetasizer Nano S. The concentration of the measured sample is twice the critical micelle concentration of the surfactant. Surfactant critical micelle concentration (mM) z average diameter (nm) polydispersity index The sensitivity of the NIBS optics used in the Zetasizer Nano Series ensures rapid determination of surfactant micelles without the need for high power lasers. To read the full article, please click on the following link: http:// Seeds,Palay Seeds,Hybrid Palay Seeds,Palay Seeds Variety XIKE AGRICULTURAL GROUP CO . .LTD. , https://www.laoseed.com
Surfactants are a class of molecules with surface active properties. This behavior is due to its amphiphilic structure, ie with both polar/hydrophilic and non-polar/hydrophobic ends [1].
• Ions (anions and cations)
• Nonionic • Amphiphilic Surfactant (Zwitterionic)