Microbial group targeted therapy development status 13 treatments in clinical development
Microbial group targeted therapy development status 13 treatments in clinical development
April 23, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];GlobalData, a world-renowned pharmaceutical industry research organization, recently released the report "Microbiome-Targeted Therapeutics in Immunology". The report points out that small biotech companies currently dominate the microbiome targeted therapy pipeline, while large pharmaceutical companies, such as Johnson & Johnson, are working eagerly with these small biotech companies and academic research institutions to further advance the development of microbial targeted therapies in the field of immunology. .
The report focuses on the development of targeted therapies for Category 2 microbial groups, a group of targeted skin micro-organisms for the treatment of skin diseases such as acne, atopic dermatitis, psoriasis, etc.; another class of targeted gut microbiome For the treatment of intestinal inflammatory diseases such as Crohn's disease, ulcerative colitis and irritable bowel syndrome.
The report pointed out that changes in the interaction between the human body and microorganisms or changes in the proportion of bacterial species may have an important impact on human health, from metabolism to immune function. So far, research on the human microbiome has not produced relevant therapies, so there is ample opportunity for pharmaceutical companies to enter the field of microbiology.
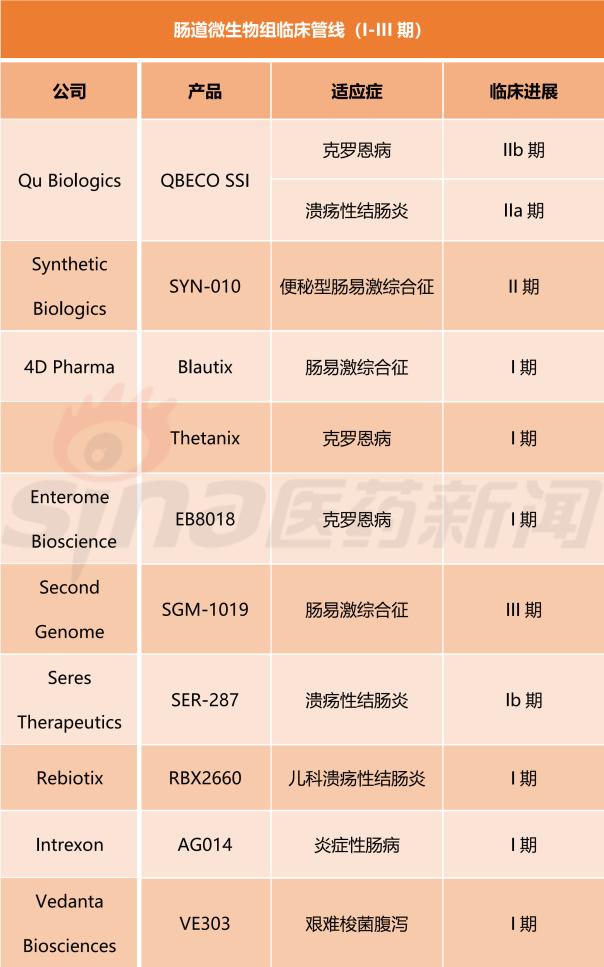
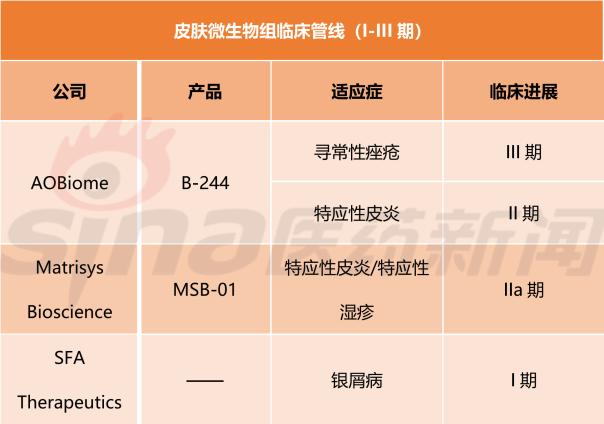
According to the report, as of the first quarter of 2018, a total of 10 therapies targeting the gut microbiome were in clinical development (Phase I to IIb/III), compared to only 3 therapies targeting the skin microbiome. In clinical development (Phase I to III).
Currently, Canadian biotechnology company Qu Biologics dominates the gut microbiome group, while the US biotechnology company AOBiome dominates the skin microbiome group. The former microbial group targeted therapy QBECO-SSI treatment of Crohn's disease has been in Phase IIb clinical development, treatment of ulcerative colitis has been in Phase IIa clinical development; the latter microbiome targeted therapy B-244 treatment of acne has been in III Phase clinical development and treatment of atopic dermatitis have been in phase II clinical development.
GlobalData pharmaceutical analyst Alexandra Annis said that Qu Biologics and AOBiome are the only biotech companies with early and late development of microbiome-targeted therapies, but these companies are hoping for the success of a drug, so they have considerable Big risk.
Key opinion leaders (KOLs) interviewed by GlobalData pointed out that pipeline therapy targeting the gut microbiome has great potential, but due to lack of scientific knowledge, many challenges have to be overcome to obtain approval. In addition, KOLs also said that the potential for fecal flora transplantation (FMT) for the treatment of inflammatory bowel disease and irritable bowel syndrome is also very encouraging.
GlobalData expects that there are plenty of opportunities to target the skin microbiome because only three line therapies are currently in clinical development: AOBiome B-244 is in Phase II/III, Matrisys Biosciences MSB-01 is treating Atopic Dermatitis Phase IIa, SFA Therapeutics' drug treatment for psoriasis is in Phase I.
Alexandra Annis also pointed out that FMT represents a very promising treatment option for the future. However, there is still a long way to go before standardizing procedures and obtaining regulatory approvals. If FMT becomes more streamlined and widely used, and its long-term security is confirmed, it may affect the sales of existing products already on the market.
The following are the 10 gut microbiota targeted therapies and 3 skin microbiome targeted therapies mentioned in the report for clinical development (stages I-III). (Sina Pharmaceutical Compilation/newborn)
Greenhouse Plastic Plastic Clamps
Greenhouse Plastic Plastic Clamps,Greenhouse Structure Connecting Clamps,Agricultural Greenhouses Clamps,Plastic Clamps
JIANGSU SKYPLAN GREENHOUSE TECHNOLOGY CO.,LTD , https://www.engreenhouse.com