CFDA and the National Health and Family Planning Commission jointly issued the medical representative filing documents. The medical representatives are not drug sales personnel.
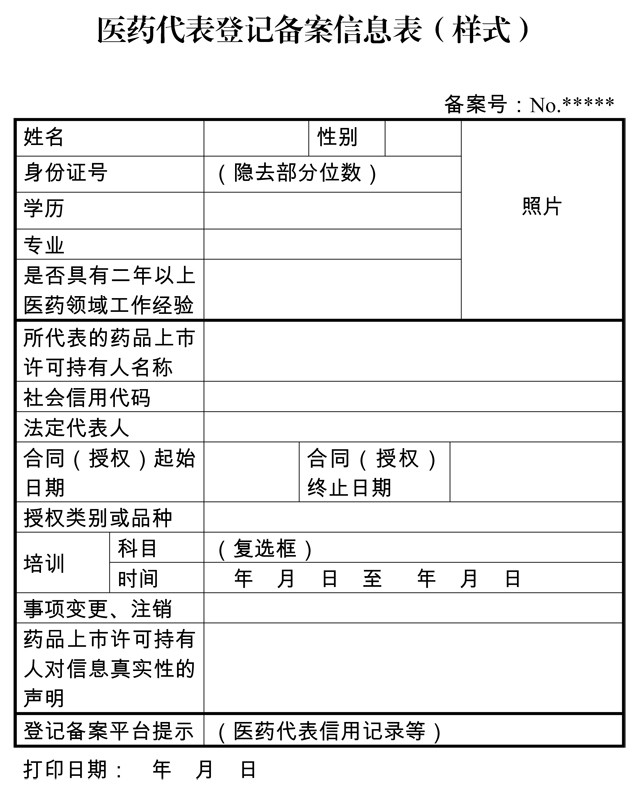
Today, the General Office of the Food and Drug Administration and the General Office of the National Health and Family Planning Commission jointly issued a public consultation for the "Administrative Measures for the Registration of Medical Representatives (Trial) (Consultation Draft)". The medical representatives in the eyes of everyone have been further clarified. Pharmaceutical representative is not a drug salesperson Article 2 of the "Draft for Comment" stipulates that "pharmaceutical sales personnel are not medical representatives and are not included in the management of these Measures." At the same time, Article 2 of the "Draft for Comment" also clarifies that "the medical representatives referred to in these Measures refer to the marketing authorization for drugs. The holder (the enterprise holding the drug approval number, the same below) is a professional who is engaged in drug information transmission, communication and feedback within the territory of the People's Republic of China. The general agent of imported drugs can represent the holder of the overseas drug marketing license. Registration and registration management work." What is the job of the medical representative? The specific contents of the pharmaceutical representative's activities include: academic promotion, technical consultation, assisting medical personnel in rational drug use, collecting and feedback on the clinical use of drugs and adverse drug reaction information. How should medical representatives and medical staff communicate? Medical representatives can communicate with medical staff in the following ways: (1) Communicating in person at a medical institution; (2) Holding academic conferences and lectures; (3) Providing academic materials; (4) Communicating via the Internet or by telephone conference; (5) Other forms agreed by the medical institution. Before engaging in academic promotion activities, the applicant for drug marketing permission should apply to the medical institution (or issue an invitation to participate in the out-of-hospital activities) and obtain approval from the medical institution before proceeding. The medical representative shall download and print the personal filing information in the registration filing platform and submit it to the medical institution for verification. What conditions should medical representatives have? (1) Junior college (including higher vocational education) or above in life sciences, medical and health, and chemical and chemical related fields; (2) Those with non-above or above qualifications shall have a college degree or above and have at least two years of working experience in the medical field. At the same time, the holder of the drug marketing license should sign a labor contract or authorization with the medical representative. The labor contract or power of attorney should indicate that the job is a medical representative; the medical representative must undergo pre-job business training to meet the drug listing permit. The person's ability requirements for the medical representative. How to represent the medical representative The holder of the drug marketing license is the subject of registration and filing, and the medical representatives employed (or authorized) shall be registered and filed on a unified platform in accordance with these Measures. The registration and filing platform for medical representatives shall be designated by the State Food and Drug Administration. The platform publicizes the registration and registration information of medical representatives, as well as the information of enterprises or medical representatives who violate the regulations (or lose their trust). The holder of the drug marketing license shall fill in the registration and registration information of the medical representative on the registration and filing platform, and the medical representative who completes the registration and filing shall be assigned the record number by the registration and filing platform. What information should be filled in by the medical representative? (1) the name, gender and photo of the medical representative; (2) Academic experience in education, professional and medical fields; (3) The ID number of the medical representative; (4) the date of commencement and termination of the labor contract or power of attorney; (5) The completion of training by medical representatives (including training subjects and training time); (6) The type or variety of drugs that the medical representative is responsible for promoting; (7) The name, social credit code and legal representative name of the holder of the drug marketing permit; (8) The authenticity statement of the holder of the drug listing permit on all the filing information. The holder of the drug marketing license is responsible for reviewing, entering, changing, and canceling the information, ensuring the authenticity of the registered filing information, responsible for the business management of the employed (or authorized) medical representatives, and conducting integrity education and business training for medical representatives. To ensure that their business practices comply with relevant regulations. Cancellation of medical representative filing information If the medical representative no longer engages in related work or terminates the labor relationship or terminates the authorization, the holder of the drug marketing license shall complete the registration and registration of the medical representative within 20 working days. If the holder of the drug marketing license is disqualified, the medical representative information of the record shall be automatically invalidated from the date of cancellation of the qualification. If the pharmaceutical representative is engaged in drug sales The "Draft for Comment" clarifies that pharmaceutical representatives may not undertake the task of drug sales, may not participate in the number of prescriptions prescribed by the statistician, may not directly sell physical medicines, may not collect and process purchase and sales bills, may not engage in commercial bribery, and shall not be in medical and health institutions. Departments and individuals shall directly provide donations and sponsorships; they shall not mislead doctors to use drugs, and shall not exaggerate or mislead the effects of drugs, and may not conceal adverse drug reactions. If the medical representative violates the above provisions, the registration filing platform shall publicize the violations according to the investigation results of the food and drug supervision department or the health and family planning department, and notify the personal credit management department; the food and drug supervision department shall order the drug marketing license holder to The medical representatives will implement the off-the-job training, and the off-the-job training will last for at least one month. If the behavior of a medical representative violates the provisions of laws and regulations, the responsibility shall be investigated in accordance with relevant laws and regulations. The holder of the drug marketing license shall not encourage or imply that the medical representative engages in violations, and shall not assign the drug sales task to the medical representative. The medical representative or other personnel shall not be required to count the number of prescriptions issued by the doctor, and may not compile the training in the registration or Deliberately provide other false information. If the holder of the drug marketing license violates the above provisions, the registration filing platform shall publicize the violations and notify the credit management department according to the investigation results of the food and drug supervision department; if there are violations of the Laws and Regulations such as the Anti-Unfair Competition Law, The relevant departments shall be handed over to the relevant departments according to law. What is wrong with medical institutions? The "Draft for Comment" stipulates that medical institutions shall not allow undocumented personnel to carry out academic promotion and other related activities within medical institutions. Medical personnel shall not participate in academic promotion activities such as approval without the approval of medical institutions, and may not accept social donation subsidies in violation of regulations. The number of prescriptions prescribed by doctors shall be provided to medical representatives or related enterprise personnel, and the use of drugs shall not be purchased in violation of regulations. Description of "related majors" in the registration conditions “College of Life Science, Medicine and Health, Chemistry and Chemical Engineering (including higher vocational education) and above†refers to the category of medical and health products and pharmaceutical manufacturing in the Catalogue of Higher Vocational Education (Specialties) in the General Higher Education (2015). Degree, chemical biotechnology, pharmaceutical biotechnology major (including higher vocational education); "general higher education undergraduate professional catalog (2012)" in the medical category, chemical, biological sciences, chemical and pharmaceutical, biomedical engineering Bachelor degree or above in engineering or bioengineering. In addition to the above-mentioned majors, other majors related to medicines (required subjects in the compulsory subjects) may be indicated in the registration filing information, such as "Management (medicine related)". Medical Representative Registration and Recording Management Measures (Trial) (draft for comments) Chapter I General The first is to regulate the practice of pharmaceutical representatives and promote the healthy and orderly development of the pharmaceutical industry. According to the Notice of the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council on Printing and Distributing the Opinions on Deepening the Reform of Examination and Approval System and Encouraging Innovation in Pharmaceutical Medical Devices (Dongzi [2017] No. 42) and the "Several Opinions of the General Office of the State Council on Further Reforming and Perfecting the Policy of Drug Production, Circulation and Use" (Guo Ban Fa [2017] No. 13), and formulate these Measures. Article 2 The term "pharmaceutical representative" as used in these Measures refers to a professional who carries out drug information transmission, communication and feedback within the territory of the People's Republic of China on behalf of the holder of the drug marketing license (the enterprise holding the drug approval number, the same below). The general agent of imported drugs may represent the holder of the overseas drug marketing license to be responsible for the registration and management of the medical representative. Drug sales personnel are not medical representatives and are not included in the management of these measures. Chapter II Employment Content and Qualifications Article 3 The specific contents of the medical representative's activities include: academic promotion, technical consultation, assistance to medical personnel in rational use of drugs, collection and feedback of clinical use of drugs and adverse drug reaction information. Medical representatives can communicate with medical staff in the following ways: (1) Communicating in person at a medical institution; (2) Holding academic conferences and lectures; (3) Providing academic materials; (4) Communicating via the Internet or by telephone conference; (5) Other forms agreed by the medical institution. Before engaging in academic promotion activities, the applicant for drug marketing permission should apply to the medical institution (or issue an invitation to participate in the out-of-hospital activities) and obtain approval from the medical institution before proceeding. Article 4 The registration of medical representatives shall have one of the following conditions: (1) Junior college (including higher vocational education) or above in life science, medical and health, chemical and chemical related majors (see the description of “related majors†for details); (2) Professional degree (including higher vocational education) or above other than (1), and have more than two years of working experience in the medical field. The holder of the drug marketing license shall sign a labor contract or power of attorney with the medical representative. The labor contract or power of attorney shall state that the job position is a medical representative; the medical representative shall undergo pre-job business training to satisfy the drug listing permit holder. The job ability requirements of the medical representative. Article 5 The holders of drug marketing licenses shall conduct business training for medical representatives, set post competency requirements and training subjects, and faithfully write training records. The holder of the drug marketing license may entrust the social institution to conduct business training, and the holder of the drug marketing license is responsible for the authenticity of the training record. The training subjects should include laws and regulations, professional ethics education, medical professional knowledge, product related knowledge and so on. Encourage relevant social institutions to develop detailed training standards or programs to provide high-quality medical representative training to drug listing license holders. Chapter III Registration and Filing Information Article 6 The holder of a drug listing permit is the subject of registration and filing, and the medical representatives employed (or authorized) shall be registered and filed on a unified platform in accordance with these Measures. Article 7 The registration and filing platform for medical representatives shall be designated by the State Food and Drug Administration. The platform publicizes the registration and registration information of medical representatives, as well as the information of enterprises or medical representatives who violate the regulations (or lose their trust). The holder of the drug marketing license shall fill in the registration and registration information of the medical representative on the registration and filing platform, and the medical representative who completes the registration and filing shall be assigned the record number by the registration and filing platform. The specific operational rules for the registration and filing platform will be announced separately on the platform. Article 8 The registration and filing platform may be constructed and maintained by relevant social organizations, but may not charge fees to drug listing license holders, medical representative individuals, and medical institutions. Article 9 The registration and filing information that the holder of the drug marketing permit should fill in on the platform includes: (1) the name, gender and photo of the medical representative; (2) Academic experience in education, professional and medical fields; (3) The ID number of the medical representative; (4) the date of commencement and termination of the labor contract or power of attorney; (5) The completion of training by medical representatives (including training subjects and training time); (6) The type or variety of drugs that the medical representative is responsible for promoting; (7) The name, social credit code and legal representative name of the holder of the drug marketing permit; (8) The authenticity statement of the holder of the drug listing permit on all the filing information. Article 10 The record information of (1)-(8) listed in Article 9 of these Measures (the number of digits of the personal identification number is hidden) and the record number shall be disclosed to the public. The medical representative shall download and print the personal record information in the registration filing platform and submit it to the medical institution for verification. The food and drug regulatory authorities and the health and family planning department have the authority to examine all of the above information. Article 11 If the medical representative no longer engages in related work or terminates the labor relationship or ceases authorization, the holder of the drug marketing license shall complete the registration and registration of the medical representative within 20 working days. If the holder of the drug marketing license is disqualified, the medical representative information of the record shall be automatically invalidated from the date of cancellation of the qualification. Article 12 The food and drug supervision department may organize irregular registration verification of the registration and filing information of the medical representatives of the listed license holders in the administrative region, and the medical representatives and related enterprises shall cooperate with the verification. The food and drug supervision department may entrust a social group responsible for the operation and maintenance registration and filing platform to conduct information verification. Chapter IV Requirements for Employment Article 13 Pharmaceutical representatives shall truthfully provide registration and filing information and practice in accordance with the law. The employment activities of medical representatives in medical institutions shall be conducted in public and comply with the relevant regulations of the health and family planning department. Article 14 The holder of the drug marketing license is responsible for reviewing, entering, changing, and canceling the information, ensuring the truthfulness and accuracy of the registered filing information, and being responsible for the business management of the medical representatives employed (or authorized), and carrying out the integrity of the medical representatives. Education and business training to ensure that their business practices comply with relevant regulations. Article 15 Medical representatives shall not undertake the task of drug sales, shall not participate in the number of prescriptions prescribed by the medical doctors themselves, shall not directly sell physical medicines, shall not collect and process purchase and sales bills, shall not engage in commercial bribery, and shall not establish departments within the medical and health institutions. Donate directly to donate funds for sponsorship; do not mislead doctors to use drugs, do not exaggerate or mislead the effects, and do not conceal adverse drug reactions. Where the medical representative violates the above provisions, the registration and filing platform shall publicize the violations according to the investigation results of the food and drug supervision department or the health and family planning department, and notify the personal credit management department; the food and drug supervision department shall order the drug marketing license holder to The medical representatives will implement the off-the-job training, and the off-the-job training will last for at least one month. If the behavior of a medical representative violates the provisions of laws and regulations, the responsibility shall be investigated in accordance with relevant laws and regulations. Article 16 The holder of a drug marketing permit shall not encourage or imply that the medical representative engages in violations, and shall not assign the drug sales task to the medical representative. The medical representative or other personnel shall not be required to count the number of prescriptions prescribed by the doctor, and may not be registered. Fabricate training or deliberately provide other false information. If the holder of the drug marketing license violates the above provisions, the registration filing platform shall publicize the violations and notify the credit management department according to the investigation results of the food and drug supervision department; if there are violations of the Laws and Regulations such as the Anti-Unfair Competition Law, The relevant departments shall be handed over to the relevant departments according to law. Article 17 Medical institutions shall not allow undocumented personnel to carry out academic promotion and other related activities within medical institutions. Medical personnel shall not participate in academic promotion activities and other activities that have not been approved by medical institutions, and may not accept social donation subsidies in violation of regulations, and may not apply to medicines. The representative or related enterprise personnel shall provide the number of prescriptions prescribed by the doctor personally, and shall not purchase the medicines in violation of regulations. Article 18 Social associations such as trade associations and societies shall play an active role in industry supervision and self-discipline, and encourage social organizations to formulate industry norms or guidelines in accordance with these Measures, and establish supervision mechanisms, credit management mechanisms and punishment measures. Chapter V Supplementary Provisions Article 19: Explain the "related majors" in the conditions for registration and registration: "Colleges of life sciences, medical and health, and majors in chemical and chemical engineering (including higher vocational education) and above" refer to "higher vocational education (specialist) in ordinary higher education institutions). Professional Catalogue (2015) in the major categories of medical and health, pharmaceutical manufacturing, chemical biotechnology, pharmaceutical biotechnology, college (including higher vocational education); "general colleges and universities undergraduate professional catalog (2012)" Bachelor degree or above in chemical, biological sciences, chemical and pharmaceutical, biomedical engineering, and bioengineering. In addition to the above-mentioned majors, other majors related to medicines (required subjects in the compulsory subjects) may be indicated in the registration filing information, such as "Management (medicine related)". If the professional catalogue has been adjusted after the revision of the above professional titles, or if the majors in foreign universities are similar to those listed above, but the titles are different, they should be recognized as appropriate. Article 20 These Measures shall come into force on the day of the month of 2018. Attachment: Medical representative registration record information form (style) Tetanus Shot,Tetanus Vaccine,Hepatitis B Injection,Hep B Vaccine FOSHAN PHARMA CO., LTD. , https://www.fspharmaapi.com