New "navigation" of drug design - neutron crystallography
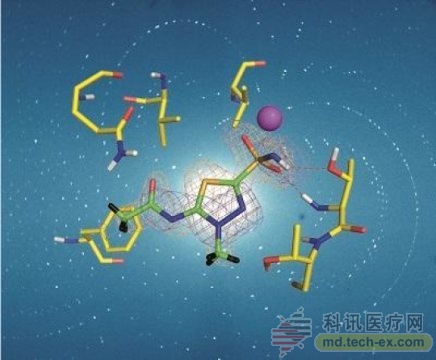
Release date: 2016-09-13 Neutron crystallography is an important complement to X-ray crystallography, which provides details of hydrogen atoms in biomolecules and the location of protons. In addition, the neutron is a non-destructive probe, and the resulting structure is radiation-free at room temperature. Hydrogen bond networks, information on the orientation and protonation state of water molecules, along with details of hydrophobic and electrostatic interactions, can help to better understand many biological processes, such as proof of important mechanisms of enzymes, and can help structure-based Guided drug design. The first neutron crystallographic study first applied to clinical drug research was acetazolamide (AZM), a sulfonamide that binds to human carbonic anhydrase isoform II with high affinity. Human carbonic anhydrase (HCA) is a zinc metalloenzyme that catalyzes the conversion of CO2 and H2O in the form of HCO3- and H. It is an important reaction to many physiological processes, including respiration, secretion and pH regulation. HCA isoforms can be used to treat a variety of diseases, such as glaucoma and epilepsy, and are prominent clinical targets. HCA II is one of 12 catalytically active isoforms, and due to sequence conservation between them, other subtypes are present with substantial off-target binding, reducing drug efficiency and causing side effects. Therefore, it is necessary to design an effective HCA-specific isotype drug. It has been determined that there are more than 400 X-ray crystals of the structure HCA II, half of which are combined with inhibitors, despite the large amount of X-ray structure data, the electrons of the H atom position and the inhibitor of the binding of proteins and solvents Key details such as status are missing for the HCA II / acetazolamide complex. In the September issue of IUCrJ [Aggarwal et al. (2016), McKenna and his colleagues described the complex of methazolamide (MZM) in the X-ray and neutron crystallographic studies of HCAII, supplementing the lack of Hydrogen bond details and hydrophobic interactions, and determine the MZM electronic state. They then compared the combination of AZM and MZM at room temperature with neutron structures and the binding of terminology and drug entropy in combination, suggesting that in the case of MZM hydrophobic forces may compensate for the lack of extensive hydrogen bonding networks. Over the past few years, more and more neutron structures have been added to the protein database, including a batch of enzyme-drug complexes. Although the overall number of neutron structures is still relatively small, there are more and more examples of using neutron crystallography to explain the problems that are still difficult to achieve using other techniques. Source: Noble Reusable Pen Injector,Cartridge Injection Pen,Injector For Self-Administrition,Customized Pen Injector Shanghai Enjosim Medical Technology Co., Ltd , https://www.enjosimmedical.com