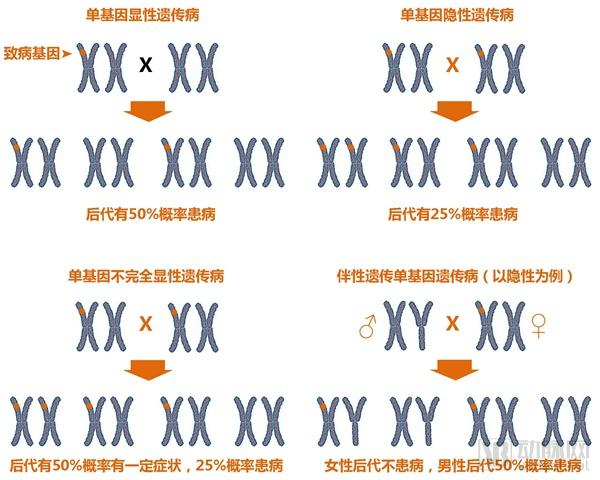
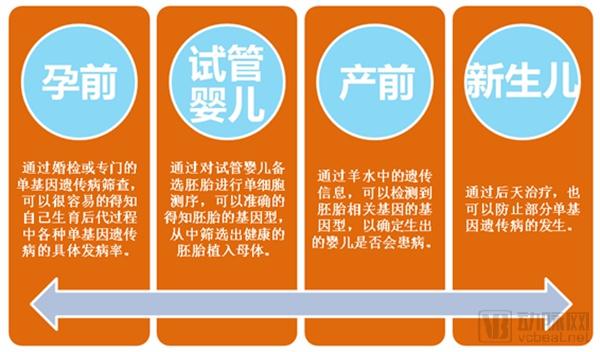
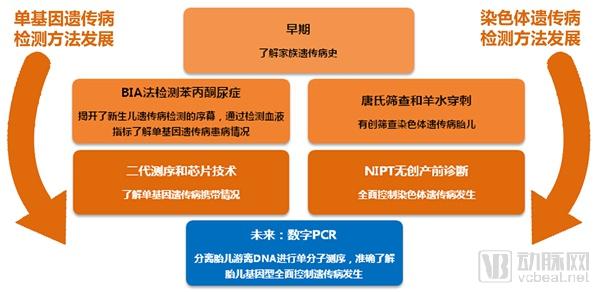
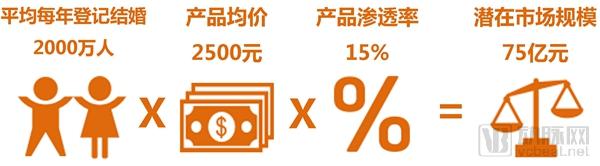
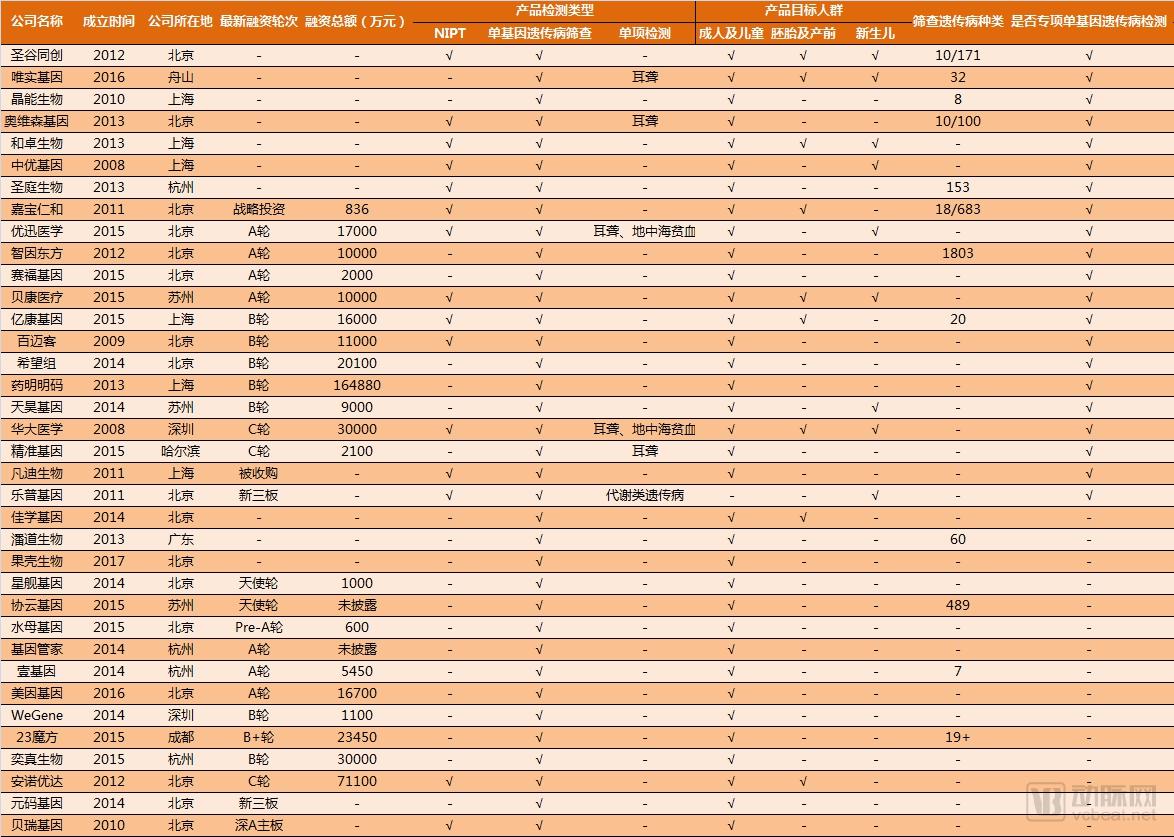
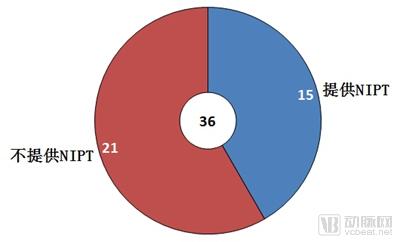
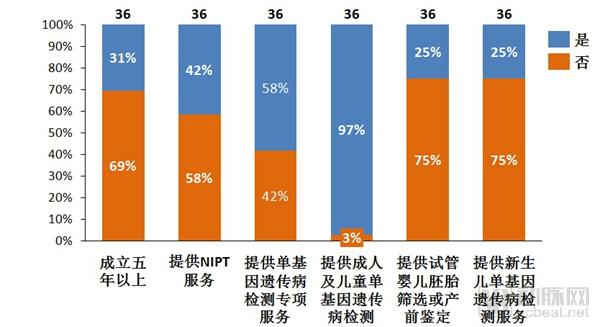
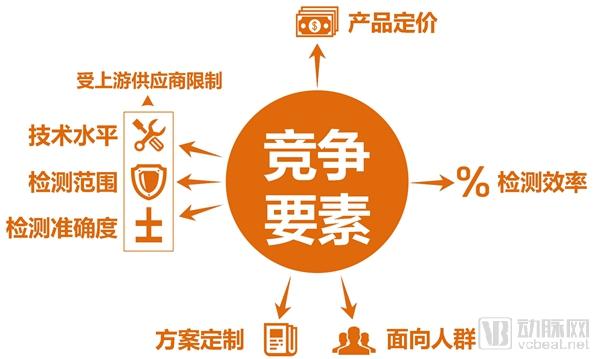
Single gene genetic disease genetic test report: the next tens of billions of market set sail
In the first three quarters of 2018, biotechnology was one of the two most active areas of global financing. In China, the NIPT industry has developed rapidly. In 2018, two listed companies were created and brought a wave of genetic testing. In the current genetic testing market, personal genetic testing and NIPT account for most of the market share. In addition to continuing to increase the number of additional products on existing products, opening new product growth points will also be a top priority. Arterial network believes that genetic testing of single-gene genetic diseases, as a relatively mature technology in the current gene sequencing industry, is likely to gain policy support in the next stage and become a new hot spot in the gene sequencing industry. Nearly 10 million people register and marry each year in China, and these newly-married couples will become the target group for genetic testing of single-gene genetic diseases, bringing tens of billions of market space. According to the monitoring of the knowledge network of the arterial network, there are currently 36 companies in China that provide different types of genetic testing services for single-gene genetic diseases, including head companies such as Huada and Berry. This is the first report in the industry specifically for the genetic testing of single-gene genetic diseases. Welcome to communicate. Report core tips: 1. The report carried out statistics on 36 domestic companies involved in genetic testing of single-gene genetic diseases. The statistics include financing situation, product type, target population, screening of genetic diseases, and price. Then, according to the statistical content, the data analysis was carried out, and the market situation of the current single-gene genetic disease genetic testing industry was described from an objective point of view. 2. The market space of the single-gene genetic disease genetic testing industry was estimated by data such as product price, target population, and estimated penetration rate. 3. According to the understanding and analysis of the industry, it puts forward important points: the marriage inspection and the newborn market deserve to be paid attention to. The following is an excerpt from the report content. One. Introduction to monogenic genetic diseases Single-gene genetic diseases refer to genetic diseases that are only regulated by a pair of alleles, also known as Mendelian genetic diseases. The classification is divided into single gene dominant genetic disease, single gene recessive genetic disease and single gene incomplete dominant genetic disease. figure 1. Genetic law of single gene genetic disease two. The importance of single genetic disease detection figure 2. Single-stage genetic disease prevention and control methods at different stages Prenatal and postnatal care has always been the top priority in China's birth policy. Single-gene genetic diseases, as a type of genetic disease that can currently be screened on a large scale, have never received sufficient attention. Many families do not conduct single-gene genetic testing with the attitude of “knowing what can be done, not yet to be bornâ€, but with the development of single-cell sequencing technology and non-invasive prenatal testing technology, most of the current monogenic genetic diseases Prevention and treatment can be carried out at various stages before, during and after pregnancy. three. Development of single gene genetic disease detection methods Figure 3: Development of a single genetic disease detection method In 1963, the BIA method for detecting phenylketonuria unveiled the prelude to the detection of neonatal genetic diseases. Subsequently, monogenic genetic diseases such as thalassemia, hearing impairment and faba bean disease also showed corresponding examination methods. The development of chip technology and the emergence of second-generation sequencing technology have brought genetic testing technology from the scientific research to the market. The second generation of sequencing technology compared to the first generation has greatly improved efficiency while reducing costs. Liquid biopsies, in turn, make the latest genetic testing technology a viable genetic testing product. Now only a few milliliters of saliva is needed, and a personalized analysis of each person's genetics can be performed. People can clearly understand whether they are carriers of a single genetic disease and what kind of disease they carry. According to the genetic test results of both husband and wife, it is easy to judge how likely the offspring will suffer from a single-gene genetic disease, and how high the probability is, so that children with monogenic genetic diseases can be effectively controlled. four. The composition and characteristics of the industrial chain of the single gene genetic disease genetic testing industry Figure 5: Single-gene genetic disease detection industry structure At present, the upstream of the single-gene genetic disease genetic testing industry is basically monopolized by Illumina, and its market share is more than 80%; Roche and Thermo products also have a certain coverage, these three large enterprises account for more than 99% of the overall market of the sequencing industry; It is divided by other foreign companies such as Oxford Nanopore and PacBio. Midstream companies purchase sequencing instruments and chips from upstream companies to provide single-gene genetic disease testing services to downstream users. Some of these companies have grown up like NIB and Berry, following the trend of NIPT, and have already had a certain size of the company, and the rest are small companies established around 2014. These companies provide different types of services for different customer groups, including personal genetic interpretation, broad-spectrum single-gene genetic testing, special single-gene genetic testing, and IVF embryo screening. Downstream users of single-gene genetic disease genetic testing are widespread. Not just adults and children, but also test tube baby embryos, prenatal pregnant women and newborns. Different types of customers have different requirements for services, and there are certain differences in specific technical means. Therefore, most companies only provide some services. Fives. Single gene genetic disease genetic testing market size The current single-gene genetic testing products are mainly for adults and children. From the perspective of preventing single-gene genetic diseases, screening of single-gene genetic diseases in newly married couples is the most critical for the prevention and treatment of single-gene genetic diseases. Let the newly-married couples who are pregnant know the harm of single-gene genetic diseases and provide them with reliable single-gene genetic disease services, which may constitute the largest market for single-gene genetic disease detection. According to the China Health Statistics Yearbook issued by the Ministry of Health in recent years and the statistical bulletin on social service development issued by the Ministry of Civil Affairs, the number of marriage registrations in China is maintained at around 10 million pairs per year, and about 20 million people should undergo premarital examinations. At present, the price of single-gene genetic disease detection products on the market is around 2,500 yuan, with a product penetration rate of 15%. It is estimated that the potential market for single-gene genetic disease detection is about 7.5 billion yuan. Figure 6: Market capacity forecast for the single-gene genetic disease detection industry In addition, the single-gene genetic testing market includes other parts such as IVF embryo screening, prenatal testing and neonatal testing. Therefore, there is still room for growth in the potential market size of 7.5 billion, which is expected to exceed 10 billion. six. Single gene genetic disease genetic testing market pattern Figure 7: Statistics of 36 companies providing genetic testing services for single-gene genetic diseases in China At present, many companies in the domestic market are providing genetic testing services for single-gene genetic diseases. The arterial network counts 36 companies currently providing genetic testing for single-gene genetic diseases and analyzes the overall data. Figure 8: Single-gene genetic disease genetic testing company provides NIPT services Fifteen of the 36 companies offer NIPT services, accounting for 41.7% of the total. There are 11 companies that have been established for more than five years, 9 of which provide NIPT services at the same time, accounting for 81.8% of the total; 25 companies established within five years (2013 and beyond), and only 6 provide NIPT services, accounting for 6 24.0% of the total. Figure 9: Service status of single-gene genetic disease genetic testing companies Twenty-one of the 36 companies offer specialized testing for single-gene genetic diseases, and 15 companies provide integrated consumer-grade genetic products. Single-gene genetic testing is only one of them. Some companies provide special single-gene genetic disease carrying conditions. These companies are rarely directly oriented to the C-end, and most of them work with hospitals to sell products. In addition to facilitating the promotion of cooperation with hospitals, because such tests require blood samples, most users cannot operate on their own and need hospital assistance. Therefore, this type of company is not very popular on the Internet, its social visibility is low, and it is more inclined to develop new testing products to broaden its business scope. In addition, the price of special single-gene genetic disease genetic testing products is relatively high, about 2,500 yuan, but the test content is comprehensive, usually covering hundreds or even thousands of single-gene genetic diseases. Another part of the company is profitable by providing personal genetic testing services, such as 23 Rubik's Cube and WeGene. Unlike special single-gene genetic testing, personal genetic testing services only need to provide saliva samples. Therefore, companies like 23 Rubik's Cube and WeGene are mostly directed to C-end users. The choice of test samples also determines the low accuracy and small coverage of single genetic disease in individual genetic testing products, and can only be used as a reference. If you want to get a more accurate genetic situation, you still need to carry out a special single-gene genetic disease carrying test. Seven. Single gene genetic disease genetic testing market competition elements Among the competitive factors in the genetic testing market for single-gene genetic diseases, there are common competitive factors such as price and technology, as well as unique competitive factors for people, efficiency, and accuracy. In our research, we classify the competitive factors related to the company's hard power into the horizontal dimension, and the competitive factors related to the company's market strategy fall into the vertical dimension, and further discuss the issue of the competitive factors in the single-gene genetic disease market. Figure 10: Competitive factors in the single-gene genetic disease testing industry In the vertical dimension, companies have large differences in performance. In the customization of the program, some companies choose to provide personal genetic interpretation products containing single-gene genetic disease detection, which we can call "bundled" sales products; while others choose to provide independent single-gene genetic disease genetic testing products. The price of bundled products is relatively cheap, such as the price of 23 Rubik's cube products is as low as 399 yuan; and the independent products that provide a wider range of detection, the price will be higher, generally around 2,500 yuan. Most bundled products are only for adults and children; while stand-alone products are detailed and have products for different subjects such as IVF embryos, prenatal fetuses, and newborns. Companies vary from market to service depending on the services offered. In a small direction, a small range of competition is carried out. Eight. Monogenic genetic disease future market development The current market development of single-gene genetic disease genetic testing focuses on adult and child and IVF embryo screening, and is insufficient for prenatal diagnosis and neonatal market development. The diagnosis of prenatal single-gene genetic diseases has not reached the level of large-scale commercialization at the technical level, so the newborn market may become the next market for genetic testing of single-gene genetic diseases. For neonatal testing, working with hospitals and month centers may be the best means of competition. Neonatal genetic testing in hospitals is still based on the old method of blood index testing. Compared with blood testing, genetic testing requires less sample size, high detection efficiency, high accuracy, and the price can be accepted by most people, which can completely replace the blood index detection method. With the deepening of genetic research and the advancement of sequencing technology and PCR technology, disease-related genetic testing products may become the mainstream of the market. At present, many companies are carrying out disease susceptibility gene detection products such as tumor susceptibility genes and cardiovascular disease susceptibility genes. When disease-related gene detection develops to a certain extent, integrated disease gene detection products will emerge. This type of more integrated product has the potential to dominate the market. nine. Single gene genetic disease genetic testing company case 1. Huada Medicine company profile company name Established Latest financing round Total financing NIPT Single genetic disease detection Adults and children Embryo/prenatal Newborn Huada Medicine 2008 C round Â¥300M √ √ √ √ Financing process Financing round Financing time Financing Amount investor Undisclosed 2010.01 Undisclosed Sequoia Capital China Undisclosed 2014.01 Undisclosed Opening investment, sharing investment A round 2014.05 Â¥100M Softbank China, Jinglin Investment, Shenzhen Venture Capital B round 2014.05 Â¥100M Tongchuang Weiye C round 2014.06 Â¥100M Songhe Capital, Shenzhen Venture Capital Founded in 2008, Huada Medical is a medical testing service company under the Huada Gene Group. The company has completed a total of five rounds of financing, of which the first two rounds of financing have not been disclosed. The following three rounds of A, B and C occurred in May-June 2014, with a financing of 100 million yuan per round. As one of the most well-known bio-tech enterprises in China, Huada has always been at the forefront of China's gene sequencing industry. So in 2008, Huada Medicine was established, and Huada Gene began to provide genetic testing services to the public. According to the semi-annual report of Huada Gene in the first half of 2018, Huada Gene achieved revenue revenue of 1.141 billion yuan in the first half of 2018, of which income from reproductive health services reached 622 million yuan, accounting for 54.5% of total revenue. %. The reproductive health content is still the main source of income for the Huada gene, and the reproductive health services include NIFTY and a variety of single-gene genetic disease genetic testing products. Product situation product name Applicable group Test sample product type product price Screening for carriers of monogenic genetic diseases Adults and children saliva Broad spectrum detection 2600 Thalassemia genetic testing Adults and children, prenatal, newborn blood Single test unknown EmbryoSeq-PGD preimplantation single gene genetic disease detection Test tube baby embryo Embryonic single cell Broad spectrum detection unknown Single gene genetic disease gene detection Adults and children saliva Broad spectrum detection unknown Neonatal deafness gene detection Newborn blood Single test unknown An Xin can be genetic testing for newborns and children Children, newborns blood Personal genetic testing 6800 The type of single-gene genetic disease testing offered by Huada Medical is the most comprehensive of all companies. The products cover the whole age, and there are corresponding single-gene genetic disease detection programs from embryo to adult. And focus on pre-pregnancy to neonatal stage, strictly control the occurrence of single-gene genetic diseases, and help eugenics. The single gene disease gene test provided by Huada Medical can detect genetic mutations involving more than 3,000 diseases and more than 4,000 genes. The sequencing coverage is over 95% and the accuracy is 99%. The sample acquisition method is to obtain the target gene fragment by probe hybridization, and then use the magnetic beads to enrich the DNA hybridized with the probe. The DNA is then sequenced to know if a genetic mutation has occurred. 2.23 Rubik's Cube company profile company name Established Latest financing round Total financing NIPT Single genetic disease detection Adults and children Embryo/prenatal Newborn 23 Rubik's Cube 2015 B+ wheel Â¥234.5M - √ - - Financing process Financing round Financing time Financing Amount investor Angel wheel 2015.03 Â¥5M Chende Capital, Softbank China, Yahui Precision Medical Fund, Materia Medica Capital, Jingwei China PreA round 2016.01 Â¥7.5M German Business Singularity, Yahui Precision Medical Fund, Jingwei China, Hanwang Technology A round 2017.01 Â¥20M German Business Singularity, Yahui Precision Medical Fund, Rich Capital, Hanwang Technology B round 2017.08 Â¥40M Hanwang QiChuang, Yahui Precision Medical Fund B+ wheel 2018.03 Â¥100M Singularity capital, rich capital B+ wheel 2018.05 Â¥62M Rich capital Founded in 2015, 23 Rubik's Cube is a provider of genetic testing services for consumer-grade genetic products. Since the establishment of the Rubik's Cube, it has experienced six rounds of financing, with a total financing of more than 200 million yuan. 23 Rubik's Cube really open the popularity should be based on the price war with WeGene. In August 2017, 23 Rubik's Cube reduced the price of genetic testing from 999 yuan to 499 yuan, followed by WeGene's product price to the same level as 23 Rubik's Cube. Then in June 2018, 23 Rubik's Cube cut prices again, and the price of the product was as low as 299 yuan. Although there has been no report on the revenue of 23 Rubik's Cube, the price cuts are still very attractive for a large number of users who are vacillating. At present, the price of 23 Rubik's Cube official website has risen to 399 yuan. 23 Rubik's technical basis comes from Thermo Fisher, using sequencing instruments and custom chips from Thermo. In the Illumina monopoly industry environment, Thermo is one of the sequencing upstream suppliers that can occupy a certain market share, so the results of the 23 Rubik's Cube test still have a certain amount of gold. Product situation product name Applicable group Test sample product type product price 23 Rubik's Cube Genetic Testing Adults and children saliva Personal genetic testing Â¥399 Although 23 Rubik's Cube did not provide special monogenic genetic disease examination, in the 23 Rubik's Cube test report, there are some descriptions of the carrying of genetic diseases, including thalassemia, faba bean disease, albinism and so on. Most of the common monogenic genetic diseases are included in the species. However, for a single-gene genetic disease that may be affected by multiple genes, such as hereditary deafness, there is no relevant content in the inspection report of 23 Rubik's Cube. In general, the entire personal genetic testing product provided by 23 Rubik's Cube is more to understand people through the interpretation of genes, and to provide a basis for the formulation of personal health plans. Due to the limitation of chip detection capacity, such detection products cannot sequence all known sites and can only cover some single-gene genetic sites. Therefore, although this product also has the ability to become a carrier of single-gene genetic disease testing services, it is still inferior to the special single-gene genetic disease detection in terms of accuracy and coverage. Laparoscopic Clip Applicator and Needle Holder We're professional laparoscopic clip applicator manufacturers and
suppliers in China, specialized in providing high quality medical
instruments with reasonable price. We warmly welcome you to buy or
wholesale bulk laparoscopic clip applicator for sale here and get
quotation from our factory. Laparoscopic Clip Applier,Hem O Lok Clip Applier,Titanium Clip Applier,Clip Applicator Tonglu WANHE Medical Instrument Co., Ltd , https://www.tlvanhurhealth.com