Automatic pipetting station PP5+1 acquisition LC-MS/MS sample operation instructions
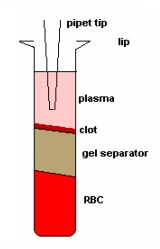
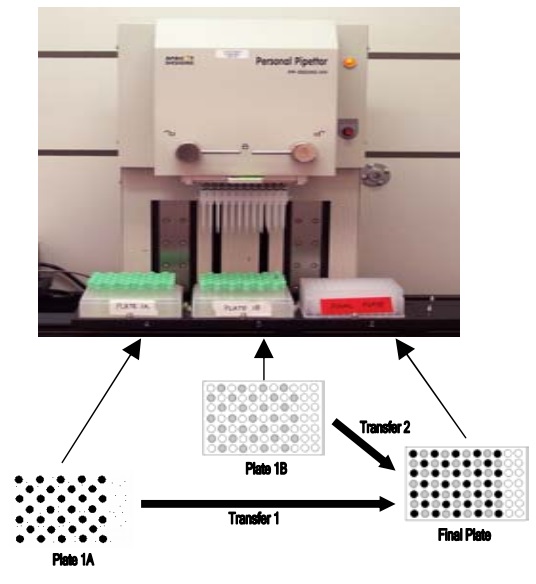
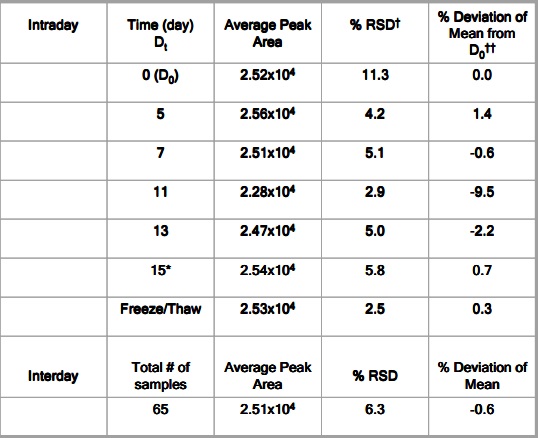
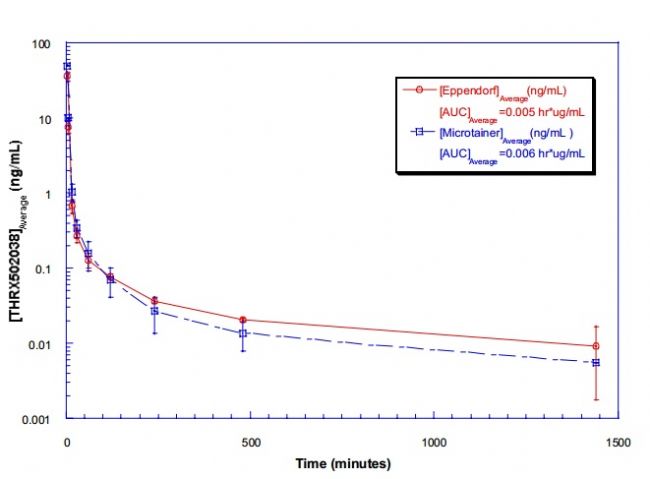
The PERSONAL PIPETTOR automatic pipetting station is a compact automatic pipetting station developed by Apricot Designs and equipped with a variety of pipetting functions. It is efficient, convenient and precise, and is compatible with 1/8/12/16/. The 24/96/384 channel liquid handling and open design make it easy to integrate stackers and robotic arms. Features and advantages 1. Combine a variety of pipetting functions in one, just click and run to complete a variety of different types of switching; The following is the collection of LC-MS/MS samples for the PERSONAL PIPETTOR automatic pipetting station . Please refer to: Overview Purpose Method Receive samples From PK→Arrange in 96-well plate→transfer samples by Apricot (See Figure 1)→Sample prep Method Column Figure 1. What is a microtainer? Microtainers are collection tubes that are used to stabilize and separate whole blood into plasma and blood cell (BC) fractions. Microtainers can contain a polymer gel separator and the walls can be coated with an anticoagulant (ie Heparin or EDTA). Benefits of using microtainers as a sample collection and storage tube: • No loss of sample • Minimal sample transfers • Minimal labeling • No decanting Figure 2. A semi-automated approach using the Apricot Personal Pipettor to transfer samples from microtainers to 96-well plates. Table 1. Statistical Analysis of THRX-776005 Stability Study in microtainers based on average concentrations. Figure 3. Stability of THRX-776005 in microtainers over a period of time. Figure 4. PK profile of THRX-502038 comparing plasma samples collected in microtainers versus samples collected in Eppendorfs. Results and Conclusion Using microtainers as a collection, preparation, and storage tube has increased sample processing productivity by 50-fold. The use of microtainers has negated the problem of plasma clots. Compound instability in the microtainer is minimal as shown in Figure 3 and defined in Table 1. Non-specific binding of compounds during routine use and storage has not been seen as shown in Figure 3. There were no great differences between using Eppendorfs (original method) and using microtainers (current method) to store plasma samples as shown in Figure 4. To date >20,000 samples have been processed using this method. The use of microtainers with heparin has shown no evidence of MS signal suppression of our compounds. (Data not shown) pain relief device, physiotherapy equipment, Other household physical therapy device Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.syztreatment.com
2. 5 function board positions + 1 needle washing tank / waste tank;
3. 1/8/12/16/24/96/384 channel pipetting;
4. Personal Pipettor+ unattended, can automatically change the tip;
5. Simple and intuitive iPad/Microsoft SurfaceTM/PC operation interface;
6. Option to use wired or wireless control of the instrument;
7. Suitable for pipetting and progressive/column gradient dilution of 96/384 well plates;
8. The three-in-one pipetting station, lock and EZ-Load tip are well sealed to ensure accurate, reliable and repeatable experiments;
9. Can be equipped with a needle washing system / barcode scanning / oscillator / temperature control module;
10. It can integrate microplate stacker and robot to realize automatic unattended operation;
11. Small size, maximum space saving, suitable for most standard fume hoods.
• Show a new and innovative way to collect, store, and prepare plasma samples using microtainers.
• Show that the use of microtainers has saved considerable time and effort in both the Pharmacokinetics (PK) and Analytical groups.
• Show that there are no compound stability or non-specific binding issues with the compounds tested by storing plasma samples in microtainers over long periods of time.
•Show that using microtainers has minimized clotting issues in plasma samples.
PK
Animal dosing→collection in microtainer→ centrifuge→store in freezer
Analytical
Sample preparation
100 uL plasma sample
+ 10 μL internal standard
+ 400 μL acetonitrile to precipitate the proteins
- 450 μL of the supernatant
Dry down under nitrogen
Reconstitute with 100 μL of 5% methanol
Higgins TARGA, C18 (30 x 0.5mm, 5 μm)
Inject 10 μL
Representative Method
Initial 0.5 minute hold followed by linear gradient of 25-85% B
Over 1.5 minutes
100 μL/minute flowrate
Mobile Phase A: 0.25% Formic Acid in Water
Mobile Phase B: 0.25% Formic Acid in Methanol
Instrumentation and Hardware
PE Sciex API 3000 using TIS
HTS PAL LEAP Autosampler
Shimadzu Pumps and System Controller
Data processed with Analyst version 1.3