Analysis of cardiomyocyte contraction using SoftMax Pro software
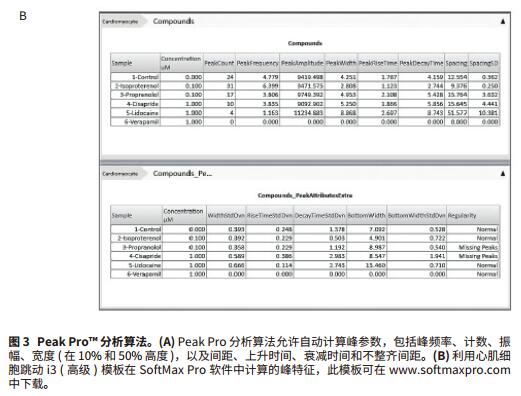
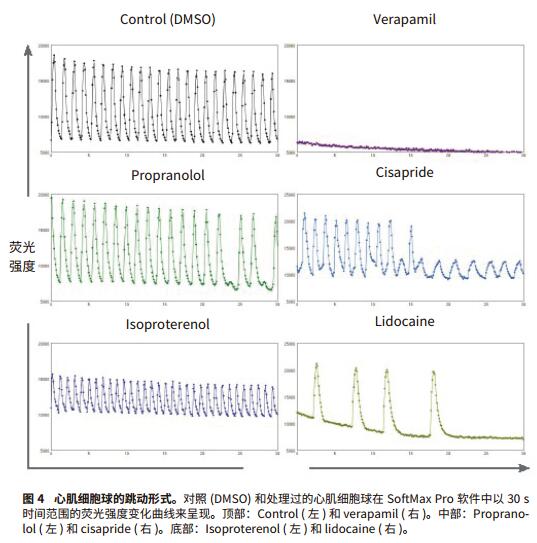
Foreword Cell-based compound screening models have become more complex to demonstrate the complexity of biological systems. Live cell imaging and three-dimensional models provide valuable insights into the structure and cellular processes of living cells. Much progress has been made in the development of complex organoidal models representing different types of human tissues, including liver, pancreas, nerves and myocardial tissue. Cardiomyocyte spheres are able to contract in an in vitro environment and respond to the effects of different regulatory factors. Here, we will introduce an experimental method including imaging and analysis, which can be used to monitor the beating of myocardial cell spheres in vitro and its beating pattern characteristics. In the case of studying cardiomyocyte toxicity, real-time observation of beating behavior of cardiomyocytes will allow scientists to obtain multi-parameter results from a single experiment using transmitted light, phase contrast, and fluorescence imaging. Screening for compound toxicity in the early stages of drug development can significantly reduce the cost of late-stage drug-induced cardiovascular side effects leading to drug development failure. This has led to the development of biologically relevant, high-throughput, high-content screening tools such as cardiomyocyte spheres. High content screening produces a large amount of data that requires fast and automated analysis. The data import feature of SoftMax® Pro software allows you to analyze any scientific data, including data from high-content imaging systems (Figure 1). By introducing image-based fluorescence intensity data into SoftMax Pro software, we were able to interpret and quantify a series of myocardial cell ball beat patterns after treatment with a series of myocardial toxic compounds. Advantage - Analytical data from experimental images Materials and Method Myocardial tissue micro sphere (InSphero AG, Switzerland) in 96-well cultured rat GravityTRAP TM (InSphero AG) detecting plate. Cell spheres were first treated with calcium ion dye (EarlyTox® Cardiotoxicity Kit, #R8210, Molecular Devices) for 2 hours, then DMSO, 0.1 μM isopropyl (nor) adrenaline, 0.1 μM propranolol, 1 μM verapamil, 1 Treat with μM cisapride or 1 μM lidocaine for 30 minutes. The processed cell spheres are imaged at 10x magnification in the ImageXpress® MicroXLS system and 10 times per second in timeLapse mode for a total of 300 time points. The imaging system is a fully automatic wide field (1x-100x) microscope capable of Fluorescence, transmitted light, and phase contrast imaging of fixed or living cells, tissues, and small organs. Time-lapse images were measured and quantified by the MetaXpress® high-content image acquisition and analysis software. Dynamic FI data is exported to Microsoft Excel, organized into Excel-based import templates, and then imported into SoftMax Pro v6.4.2. Analyze data using a preset myocardial cell beating i3 (advanced) template, which can be downloaded from and automatically calculates the characteristics of 14 peaks: peak count, peak frequency, peak amplitude, average peak width, and Standard deviation, mean peak rise time and standard deviation, mean peak decay time and standard deviation, mean peak spacing and standard deviation, mean peak width and standard deviation, and peak law. result In this study, the beating cell spheres of rat myocardial microtissue from InSphero were treated with a series of cardiotoxic compounds and assayed by the EarlyTox Cardiotoxicity kit, which contains calcium-sensitive dyes for detection of myocardial cell contraction. Changes in cytoplasmic calcium concentration. The processed cardiomyocyte time-lapse image was acquired by ImageXpress MicroXLS to observe its beating behavior (Figure 2). Fluorescence intensity fluctuation results were imported into SoftMax Pro v6.4.2 using an Excel-based import function template to quantify the effects of peak parameters and compound on myocardial jitter (Figure 3a). Isoproterenol-treated adrenaline-treated cardiomyocytes had a higher frequency of ballots compared with controls, whereas cardiomyocytes treated with propranolol, cisapride, and lidocaine were reduced. In addition, in compounds with reduced frequency, cisapride can disrupt the beating pattern of cardiomyocyte spheres. Verapamil, a calcium channel blocker that treats cardiomyocyte spheres and inhibits calcium signaling (Figures 3b and 4). In summary, these results are in complete agreement with previous studies5,6 . in conclusion The use of biologically relevant, cell-based models for high-content and high-throughput myocardial toxicity screening assays can significantly reduce the time to discovery of cardiotoxic drugs in early drug development. In order to maintain the efficiency of high throughput and high content screening assays, the same rapid data analysis is required. SoftMax Pro's import capability for SoftMaxPro v6.4.1 or higher enables analysis of microplate formats from a variety of scientific instruments including imaging systems, real-time PCR systems, non-Molecular Devices readers, scintillation counters, and more. data. Two import options based on Excel and XML provide a range of automation functions, from manual import to fully automated data import and analysis. In addition, Peak Pro software algorithms and preset templates can be used to quantify fluctuations in multiple peaks such as cardiomyocytes and cardiomyocyte ball beats. SoftMax Pro's equation system and Syntax Helper can help you get the results you need, including IC 50 The result of the curve and condition pass/fail. Custom templates can be reused to ensure efficient and consistent analysis regardless of data source. references 1. Kermanizadeh A, Lohr M, Roursgaard M, et al. Hepatic toxicology following single and multiple exposure of engineered nanomaterials utilising a novel primary human 3D liver microtissue model. Particle and Fibre Toxicology. 2014; 11:56. Rhodium powder Rh≥99.95% Properties: silver-white metal, extremely hard, wear-resistant, also has considerable ductility Application: It can be used as raw material for electrical instrumentation, chemical industry and manufacture of precision alloys. Rhodium powder is based on the widespread use of ruthenium in the industrial industry. Since rhodium is a rare metal required by the industry, the industry price is slightly higher than that of general non-ferrous metals. Rhodium, one of the rare elements, has various uses. Rhodium can be used to make hydrogenation catalysts, thermocouples, platinum-rhodium alloys, etc. It is also often plated on searchlights and reflectors, and is also used as a polishing agent for gemstones. and electrical contact parts. precious metal XI AN RHINE BIOLOGICAL TECHNOLOGY CO.,LTD , https://www.rhinebiotech.com
- Rapid quantification of myocardial cell beating patterns using the SoftMax Pro Peak Pro analysis algorithm
- Analysis of raw data obtained from any other imported scientific instrument using the same software system 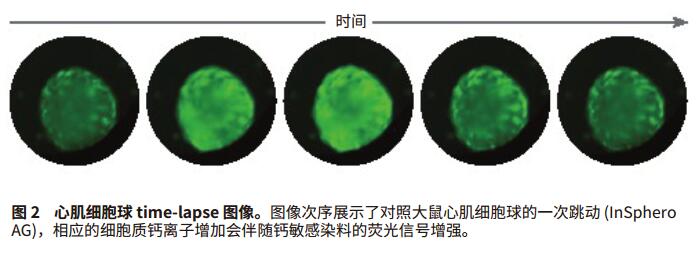

2. Zuellig RA, Cavallari G, Gerber P, Tschopp O, Spinas GA, Moritz W, and Lehmann R. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. Journal of Tissue Engineering and Regenerative Medicine. 2014 doi :10.1002/term.1891
3. Kraus D, Boyle V, Leibig N, Stark GB, and Penna V. The Neuro-spheroid-A novel 3D in vitro model for peripheral nerve regeneration. Journal of Neuroscience Methods. 2015; 246:97-105.
4. Zuppinger C, Agarkova I, Moritz W, and Kelm J. A human 3D myocardial microtissue model for cardiotoxicity testing. Poster presented at: EUROTOX 2013. 49th Congress of the European Societies of Toxicology. 2013 Sep 1-4; Interlaken, Switzerland .
5. Sirenko O, Cromwell EF, Crittenden C, Wignall JA, Wright FA, and Rusyn I. Assessment of beating parameters in human induced pluripotent stem cells enable quantitative in vitro screening for cardiotoxicity. Toxicology and Applied Pharmacology. 2013; 273(3): 500-507.
6. Sirenko O, Crittenden C, Callamaras N, Hesley J, Chen YW, Funes C, Rusyn I, Anson B, and Cromwell EF. Multiparameter In Vitro Assessment of Compound Effects on Cardiomyocyte Physiology Using iPSC Cells. Journal of Biomolecular Screening. 2013 18:39-53.
