"The fate of leachate" in upstream and downstream processes of biopharmaceuticals
Blood Lancet,Medical Blood Lancet,Disposable Blood Lancet,One Time Using Blood Lancet Yancheng Rongtai Labware Co.,Ltd , https://www.rongtailab.com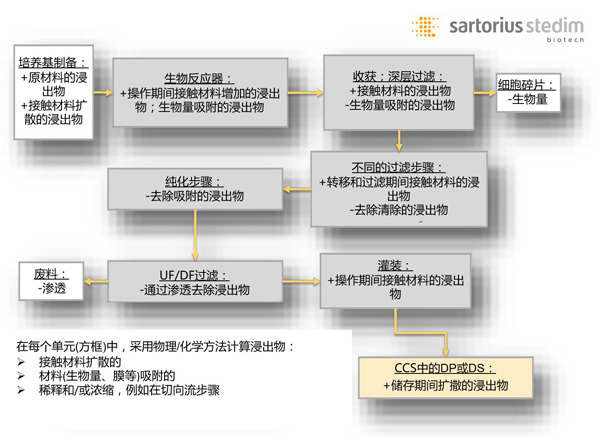
Figure 1 assumes a dynamic unit model for subsequent process steps in downstream production of biopharmaceuticals 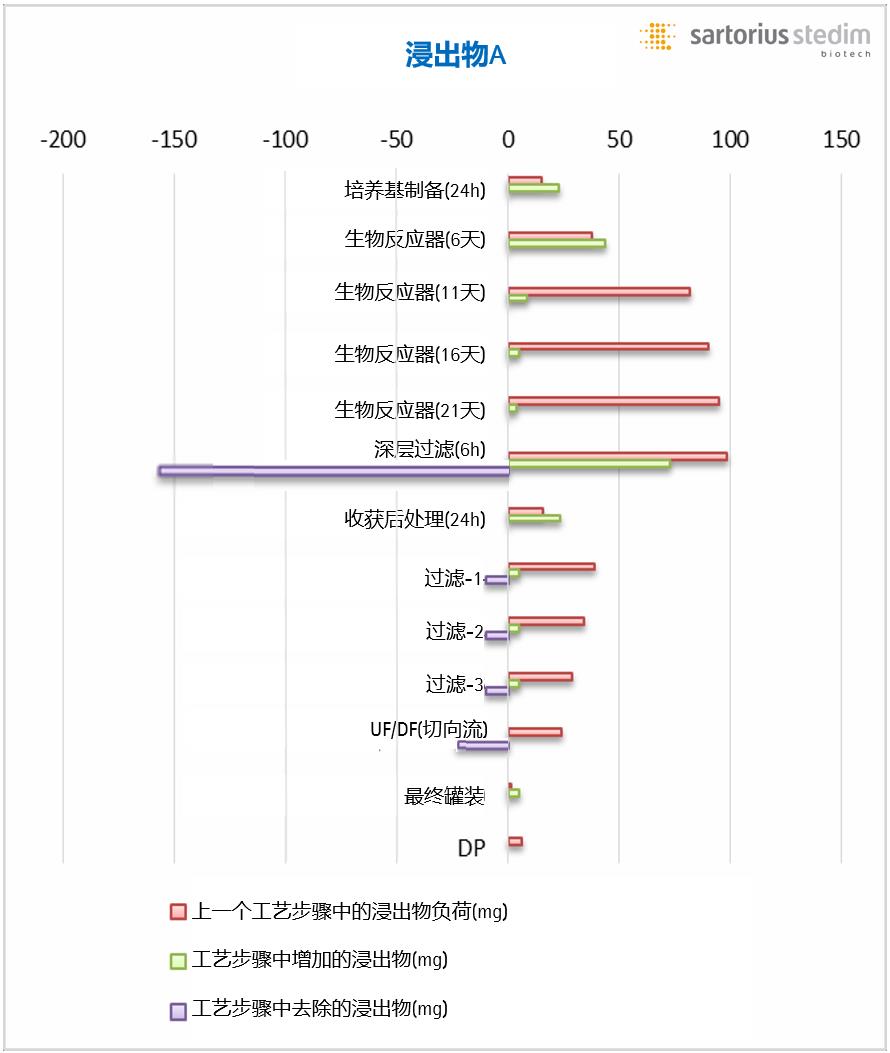

Fax: 021.6687233
Email: info. 
