HPLC Troubleshooting 3 - The emergence and settlement of Ghost Peak
We are manufacturer of First Aid Bag And Box in China, if you want to buy Trauma First Aid Bag,Medical Emergency Plastic Box,Tactical Medical First Aid Kit Case please contact us. First Aid Bag And Box,Trauma First Aid Bag,Medical Emergency Plastic Box,Tactical Medical First Aid Kit Case Medton Medical , https://www.medtonmedical.com Ghost peak problem Preventive measures and solutions Ghost peak Column or syringe is contaminated (contamination by column or injector) Use only HPLC grade solvents
Flush the column to remove impurities
When the syringe is used in the next analyte, it should be flushed first. Delayed elution peak from the previous injection (post-elution peak from previous injection) Extend the run time Flush the column with a strong mobile phase at the end of each run For a gradient run, end with a higher concentration (organic phase end gradient) Water is contaminated in RP HPLC 1. Use HPLC grade water Unknown (unknown impurity) interference in the sample 1. Use sample cleaning (purification measures) (eg SPE) Negative peak Solute refractive index is lower than mobile phase (RI detector) Use a mobile phase with a lower refractive index
Swap the detector polarity to obtain a positive peak The absorption of the solute is lower than the absorption of the mobile phase (the UV absorption of the solute is lower than the UV absorption of the mobile phase) (UV detector) Change UV wavelength
Use a mobile phase with lower UV absorption The composition of the sample solvent and mobile phase are different 1. If possible, replace the sample solvent and dissolve the sample in the mobile phase (replace the sample solvent and, if possible, dissolve the sample using the mobile phase) Peak (burr) Bubbles in the mobile phase Degas the mobile phase
At the outlet end of the detector, a back pressure limiter is installed.
Ensure that all accessories are securely installed No end caps (plugs) are used when the column is stored End caps should be used when storing the column
Rinse the RP column with degassed methanol
3.1. The unexpected broad peak in isocratic separation is the peak elution delay due to the previous run (in isocratic separation, the late eluting component in the previous run unexpectedly appeared as a broad peak).
For isocratic separation, the longer the retention time, the wider the peak (peak); however, all peaks in the narrow region of the chromatogram should have approximately the same peak width. As shown in Fig. 11 (arrow), when a broad peak appears in a narrow peak, it may be because the elution delay occurs in the compound of the previous injection (previous injection). This is easy to check.
Just do a normal injection (injection), but extend the run time two to three times. If a peak (peak) occurs after the end of normal operation time, it is caused by the elution delay.
You can extend the uptime to cover the elution of this peak (the elution time will elute the peak); you can also add a strong solvent rinse at the end of each run to clean the stubs in the column. 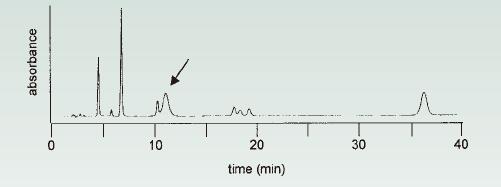
Figure 11. Peak-delayed elution usually occurs at 38.5 minutes (late elution peak (normally eluting peak at 38.5 minutes)), located at (shown in) the next shortened isocratic chromatogram 12.0 minutes (arrow). Source [6].
3.2. By running a non-injected (no injection) blank gradient and observing the baseline, the ghost peaks in the gradient run can be separated.
As shown in Figure 12a, when there are too many peaks in the blank gradient, reagent contamination may be one of the causes of this problem (probably due to solvent contamination).
In this case, Figure 12a shows that the peak (peak) during operation is very small (1-3 mAU), and there is almost no peak in the principal component analysis in the range of 0.8-1.0 AU (for a size of 0.8-1.0 AU) The main component detection peak has no effect); however, for stability (sex indicator detection) or impurity determination, peaks in the range of 1-2 mAU should be quantified (also required to be quantified).
In this case, further research is needed.
During the equilibrium between the gradient runs, non-polar impurities in the mobile phase are typically deposited (aggregated) at the top of the column. Then, during the gradient process, like other peaks, these impurities are eluted.
Check the root cause of the problem by extending the balance time to three times. If the peak (peak) in the blank gradient is increased by about three times, the aqueous solvent is most likely the cause of the problem (and most likely due to impurities in the aqueous solution).
As shown in Figure 12b, water and/or additives can be exchanged for higher purity components to solve this problem. 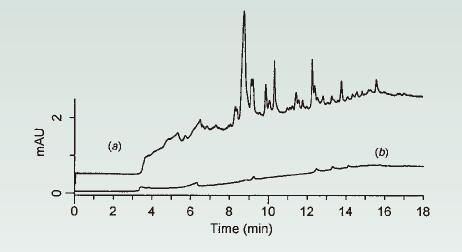
Figure 12. Running a blank gradient: (a) A container contaminated water (the solvent in the A container has impurities) and (b) a higher purity water in the A container. Column: 150 x 4.6 mm C18; 1.5 mL/min; 35 ° C; UV detection at 255 nm. Gradient: 0-83% ACN / water run for 13 minutes and hold for 5 minutes. Source [7].
3.3. Negative peaks for isocratic or gradient runs are not as common as positive peaks (relative to positive peaks), but they can also occur.
Negative peaks are more common in ion pairs or other methods where the mobile phase reagent has significant UV absorbance at selected detection wavelengths (down peaks in the presence of ion pairs or mobile phases with significant UV absorption at the detection band) More common).
In this case, background absorbance will be extremely important (background absorption may be significant) (possibly 0.5 AU or more), but will not be noticed (harder to detect) because the system will automatically start at the beginning of each run Adjust to zero and detect the signal.
If the absorbance of the compound is lower than the mobile phase background (background absorbance), it will appear as a negative peak.
The cause of confirmation and peak elimination are the same as the positive peak (the method of confirming the source of the problem and eliminating the peak is the same as the positive peak): Check the preparation process of water, reagent or sample.
