Fentanyl control, why give medical devices a great opportunity?
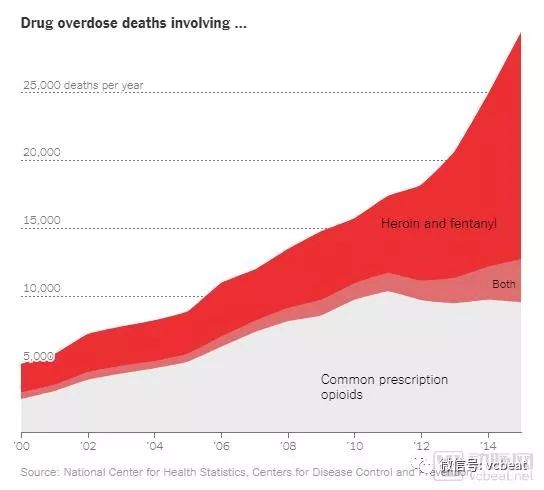
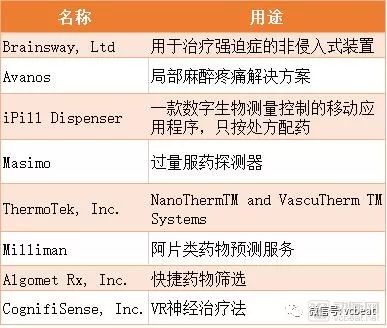
In the recent US dollar first meeting, the two sides agreed to take positive actions to strengthen cooperation in law enforcement and drug control, one of which is to control fentanyl. Suddenly, fentanyl became the protagonist of the news screen, let people re-recognize it. In the United States, the opiate crisis represented by fentanyl is no less than the impact of the proliferation of guns. Fentanyl is one of the opioids, a class of drugs similar to the alkaloids found in opium poppy, including illicit drug heroin, synthetic opioids such as fentanyl, and legally prescribed Pain medications used, such as oxycodone (OxyContin), hydrocodone (Vicodin), cocaine, morphine, and a variety of other drugs. Fentanyl has the most concern, compared to opium extracted from plants, as a drug synthesized from the laboratory, it can be a laboratory drug, it can also have better analgesic effect and less side effects. Analgesic. It is a three-point drug, and once an opioid is abused, it is equal to drugs. It is understandable to strengthen the control of fentanyl. However, the arterial network research found that this control has brought significant benefits to medical devices and digital prescription therapy. The main contents of this paper are as follows: 1. Synthetic opioid fentanyl is not wrong, poor management of prescription drugs brings crisis 2. FDA attempts to prevent opioid addiction by encouraging innovation in medical devices; better helping patients manage pain; even replacing opioids 3, blockchain, VR technology, digital prescription therapy, etc. may become a new hope to solve opioid drugs, Samsung, Novartis, Oculus and other companies are laying out. Poor management of prescription drugs boosts the spread of opioids In the United States, according to the New York Times, the number of deaths caused by overdose in 2016 exceeded the number of deaths caused by guns and car accidents. Overdose is also the leading cause of death in people under the age of 50 in the United States, mainly because of opioids. It is estimated that more than 2 million Americans have problems with opioid dependence. According to the latest survey data, more than 97 million people took prescription painkillers in 2015; 12 million of them took medication without the guidance of a doctor. In October last year, Trump signed a memorandum stating that the United States entered a national public health emergency in response to the crisis of opioids. The root cause of this crisis stems from an overdose of opioids in the United States. As you can see from the picture below, the general prescription opioids in death caused by overdose are as powerful as heroin and fentanyl. Opium addiction was in the Qing Dynasty, but the US opioid crisis began in the 1980s, and several influential journal articles allowed doctors to relax their use of opioids. The tight strings were relaxed, opioids began to flood the market, and pharmaceutical companies began to publicize. The more people, the more people start to become addicted to drugs. Some people take the initiative to try, while others start taking prescription drugs and eventually become drug addiction. Because fentanyl is also used to make fake medicines, fentanyl is difficult to be traced and searched as a synthetic drug. One of the reasons for the opioid crisis is the lack of prescription regulation. The current US prescription system relies on trust—the trust between the patient and the prescriber, the trust between the prescriber and the pharmacy, and the trust between the pharmacy and the patient. But in fact, handwritten, paper-based prescriptions are easy to forge and change. To solve the problem of the spread of opioids is to control the export of the prescription, so that the person who uses the drug can use the medicine, and the overdose is prohibited. FDA encourages medical device innovation to respond to the opioid crisis The FDA has also put a lot of effort into this area. Its initial logic is to replace opioids with different analgesics. However, no good drugs have been found to be able to achieve the replacement. With the development of digital health technology, FAD also came up with the promotion of medical device innovation to alleviate the opioid crisis. In addition, the FDA has hosted an Innovation Challenge to select digital solutions to address the opioid crisis. The challenge is to stimulate the development of medical devices , including digital health technologies and diagnostic tests, which provide new solutions for detecting, treating and preventing addiction, treating metastasis and treating pain. In this challenge, a total of 250 applications were received, and the FDA finally selected 8 products. The FDA said that although the equipment of these eight companies will not be directly marketed, they will have more interaction with CDRH experts, receive clinical trial guidance, and expedite approval. "We believe that medical devices are the most promising people to help opioid addiction, by managing pain or prevention, as an opioid alternative or to reduce opioid dependence," the FDA said in the announcement, "for example, a The development of diagnostic devices, whether in vitro diagnostic tests, software or mobile medical applications, may identify patients who need to be treated with caution." In the past few years, CDRH has approved more than 200 devices related to pain management or management (cleared, granted, approved). Of these, 10 are using new technologies, and the FDA hopes these new devices will reduce dependence on opioids. “The challenge of innovation is another example of how we bring advanced treatments, including medical devices, to individuals who need them. Collaboration with selected developers is expected to drive innovation and successfully bring these products to market.†FDA Said in the announcement. Digital health therapy will be the export of the opioid crisis The FDA is promoting medical devices to solve the opioid crisis, mainly through medical devices to better manage opioids, manage pain, and replace opioids. Attached are eight products that the FDA is optimistic about in the Innovation Challenge, which represent the direction the FDA wants to encourage in pain management. In addition, there are many digital health solutions that are equally interesting. Blockchain It is hard to imagine that blockchain can also save opioid addiction. The blockchain provides a safe and reliable prescription platform for clinicians, pharmacies, and patients to ensure clear accountability and transparency. The solution currently being developed will allow clinicians to understand their own prescription history in addition to knowing the patient's prescription history. This is because each prescription is recorded on the blockchain, allowing the government or regulatory agency to better track and combat abuse of the prescriber. VR technology Although VR has not been a slogan for the past two years, it has been optimistic about pain management and is expected to be a substitute for certain narcotic drugs. Samsung has been paying attention to this aspect and continues to improve its technology. In March 2018, Samsung announced a joint VR study on pain relief with Travelers Partners Insurance, Cedars-Sinai Medical Center, Bayer, and AppliedVR. The study relies on wearable devices and VR-assisted techniques to alleviate acute orthopedic pain and limb pain. The goal of the project is to use the most advanced technology to improve the treatment of injured workers. Chronic pain in the factory can cause a series of problems, and injured employees may use opioids to cover up the pain. The study used non-pharmacological VR treatment to help injured employees avoid chronic pain, reduce the chance of opioid addiction, and reduce medical costs. In the case of childbirth, foreign attempts have been deep, and there have been products that apply VR to pain management during childbirth. VRHealth has partnered with Oculus to use VR to alleviate the pain of cancer patients and childbirth mothers. VRHealth's non-invasive medical tools are delivered through VR content while providing advanced data analysis using artificial intelligence and cloud computing algorithms. VRHealth uses highly accurate tracking tools to provide data analysis for use in hospitals, healthcare facilities or remote environments. VRHealth works with a number of world-renowned US healthcare providers, hospitals and rehabilitation centers. VRHealth is using Oculus' products Go and Rift to provide VR technology solutions for a variety of health challenges, from pain management during maternal delivery to reducing anxiety before and after surgery for cancer patients during chemotherapy. Virtual reality takes patients to an environment where they can more actively observe and experience treatment. Through VRHealth's fascinating solutions, healthcare has become an interesting activity. APP The application rescues the crisis of opioids mainly at the front end, helping people reduce misconduct by managing drugs and identifying opioids. The FDA recently approved a mobile app that helps treat alcohol, marijuana, and cocaine addiction, and clinical trials show that 40% of patients who use the app quit addiction within three months. Of the patients who were dependent or who received standard therapy alone, only 17.6% quit addiction or dependence. This product is a product of Pear Therapeutics called ReSETTM and is the first FDA to pass an electronic drug for the treatment of drug abuse disorder (SUD). It allows patients to regain control of drug addiction and can assist in a range of stimulants, marijuana, cocaine or alcohol-dependent treatments. Although it has passed FDA certification, it has not yet been commercialized and is still being tested. Although it sounds at the forefront, Pear Therapeutics has now signed a partnership with Novartis to develop drugs. Pear Therapeutics' competitor, Click Therapeutics, also received a $17 million financing from Sanofi in July, a number that is enough to demonstrate the promise of prescription digital therapy. Click Therapeutics In addition to the commercialized product Clickotine® for smoking cessation, the company also has a wealth of research pipelines, such as CT-152 for the treatment of depression, CT-141 for the treatment of insomnia, and treatment of acute coronary artery. Digital prescription therapy such as CT-111 for syndrome and CT-130 for chronic pain. Office Safe Box,Commercial Safe Box,Heavy Biometric Office Safe Box,Large Office Safe Box Hebei Yingbo Safe Boxes Co.,Ltd , https://www.ybsafebox.com